How has robotic repair changed the landscape of mitral valve surgery?
Introduction
The principles of mitral valve (MV) repair were established soon after the development of the cardiopulmonary bypass (CPB) machine, as correction of congenital abnormalities became more routinely performed. In 1958, Dr. Dwight C. McGoon at Mayo Clinic introduced a durable technique to repair MV prolapse via a right thoracotomy approach, by reducing the flail segment using an inverting pleating stitch of 3-0 silk suture (Figure 1). He additionally closed clefts with interrupted sutures and performed commissuroplasty. Dr. McGoon’s strong emphasis on the principle of repairing the leaflet itself as opposed to using annuloplasty alone or employing an immobile prosthesis for leaflet substitution laid the foundation upon which modern repair techniques are based (1). Dr. Hartzell V. Schaff further modified the McGoon plication technique by performing triangular leaflet resection for redundant myxomatous leaflet tissue. Schaff reasoned that an unfolded McGoon plication was triangular in shape and further more, that leaflet debulking during resection enabled more symmetrical leaflet coaptation, delivering an aesthetically appealing result. He and his colleagues from Mayo Clinic subsequently demonstrated excellent long term results of posterior leaflet based repairs using triangular resection, supported with a standard length flexible posterior annuloplasty band (63 mm) anchored between left and right fibrous trigones (2,3). Standard length annuloplasty is based upon the principle that dilatation of the mitral annulus in degenerative disease primarily occurs posteriorly, and that reduction to a standard size supports coaptation and protects the leaflet repair without causing stenosis or systolic anterior motion (SAM) of the anterior mitral leaflet. Prolapse of anterior leaflet scallops is effectively addressed with artificial Gore-Tex neochordae, which have been popularized worldwide (4).
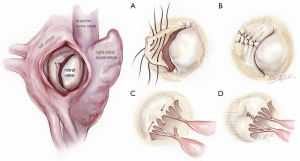
Other major contributions to present day repair techniques were made by Carpentier (5), who standardized valve analysis and MV repair using quadrangular resection and sliding leaflet plasty. He also performed the first robotic MV repair in 1998 in France (6) using an early model of the da Vinci Surgical system (Intuitive Surgical Inc., Mountain View, CA). Soon thereafter, Dr. Friedrich Mohr at the Leipzig Heart Center in Germany (7) expanded the scope of robotic cardiac surgery by performing both coronary and mitral procedures before adopting a video-assisted thoracotomy-based approach. In the USA, the first robotic MV repair was carried out by Chitwood in 2000 (8), who performed middle scallop resection and a band annuloplasty. The totally endoscopic approach, popularized by Vanermen and Casselman (9), remains the most frequent video-assisted platform in Europe. Some contend that dispersion of the thoracoscopic procedure will be impeded by the limited dexterity of long-shafted non-wristed instruments in a small space and the often-associated difficulty in visualizing the entire MV field. The robotic platform consequently evolved in order to address these challenges. A multicenter trial led to the Food and Drug Administration approval of the robotic platform for this purpose in 2005 (10,11) and several large centers have subsequently established active robotic MV repair programs. Building upon these trends, we sought to translate the principles of durable open MV repair employed at Mayo Clinic, into the closed chest milieu, utilizing robotic assistance and port-access incisions. In doing so, we developed a program capable of equally addressing all categories of leaflet prolapse, from simple to complex anterior or bileaflet pathology, with equivalent short term outcomes in comparison to propensity matched patients undergoing open operations (12).
What do North American trends show? According to a recent executive summary report of Society of Thoracic Surgeons adult surgery database, 1,041 sites reported 278,571 cardiac procedures in 2013, of which 6,554 were isolated MV replacements (MVR) and 8,814 isolated MV repair. An overall mortality of 5% was reported for MVR with a mean post-procedure length of stay of 10 days. Mortality was 1.1% for MV repair (combined approaches) with a mean length of stay of 7 days. Our own institutional experience at Mayo Clinic parallels these results with an early mortality of 0.2% with a median hospital stay of 3 days for robotic MV repair.
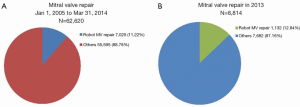
A more recent minor data request from the STS Database in 2014 for the period January 1, 2005 to March 31, 2014 demonstrates that minimally invasive techniques are becoming more frequently utilized amongst centers reporting to the Registry. Of the 62,620 total MV repair procedures performed during this period, 7,025 (11.22%) were carried out using robotic assistance (Figure 2A). Since a specific code for robotic procedures was gradually introduced, an increasingly accurate estimate of the number of robotic procedures performed annually in the USA is now available. Amongst 8,814 MV repair operations, 1,132 (12.84%) were done using robotic assistance (Figure 2B) in 2013; similar to a previously reported trend (13).
On the world stage, MV primary disease pathology can broadly be divided into degenerative disease, which is the predominant subset treated in developed nations, versus rheumatic pathology, which more often attracts surgical attention in underdeveloped nations. In those regions where degenerative MV disease is frequently treated, right chest thoracoscopic-alone and robot-assisted procedures are increasingly utilized.
The Mayo Clinic experience
The Mayo Clinic robotic MV repair program began in 2008 and has been previously described (14). All candidates for the procedure undergo preoperative computed tomography (CT) of chest, abdomen and pelvis to assess vascular disease of the aorta and ileo-femoral vessels. Contraindications for a robotic approach using peripheral cannulation at the present time include: (I) coronary arterial disease requiring open revascularization; (II) peripheral vascular disease precluding safe cannulation of the femoral vasculature; and (III) prior sternotomy or right thoracotomy. Patients are not selected or excluded based upon leaflet prolapse anatomy or complexity, due to the fact that the exact same valvuloplasty/annuloplasty maneuvers performed via an open chest approach are rigorously duplicated with robotic assistance. Utilizing a lateral 5-port approach [three in the fourth interspace for the camera, dynamic left atrium (LA) retractor and working port; right arm two spaces below and left arm two spaces above], peripheral CPB is typically established through a 1-1.5 cm incision in the right groin. Bicaval venous drainage is secured via femoral and right internal jugular vein access. The aorta is cross-clamped with a transthoracic clamp at approximately the third interspace near the mid-axillary line, and cardioplegia delivery along with left heart venting is carried out via a tack vent placed temporarily in the ascending aorta. We reviewed our initial robotic MV repair experience over four quartiles following initiation of the program, demonstrating a distinct temporal learning curve (15). We observed that by the fourth quartile, most patients were extubated in the operating room (OR) with no significant increase in total OR time. We did notice, as have others, that extubation in the OR translated into shorter intensive care unit (ICU) length of stay, lower blood product requirement (13) and total hospital stay (13,16).
We further noted a reduction in intra-operative use of opioids with a near universal pre-anesthetic placement of thoracic para-vertebral block injecting 0.5% bupivacaine with epinephrine (1:200,000) at two or three levels between T2 and T6. Because we have not modified the technical aspects of MV repair procedure itself to accommodate for a minimally invasive approach, we have routinely been able to repair all subsets of degenerative valve regurgitation involving both leaflets with 100% repair rate in over 500 minimally invasive and robotic repairs to date.
Cost considerations
We subsequently analyzed and published the economic impact of open versus robotic MV repair at Mayo Clinic during a period where both techniques were subjected to system-wide cost containment measures (17). We observed that during the initial part of our experience, there was a significantly higher cost early following introduction of robotic MV repair compared to the open, both in the OR and in the post-operative period. We quickly demonstrated however, that technical innovations such as robotics, introduced together with systems innovations including surgical process improvement, significantly decreased overall in-hospital resource utilization. For example, by facilitating extubation of patients in the OR, translating into shorter ICU stay and efficient transfer to step-down the evening of surgery, along with efficient coordination of pre-dismissal echocardiogram, our experience has been that patients are routinely prepared for discharge from hospital by the 3rd postoperative day. These trends have resulted in the overall costs of robotic MV repair becoming indistinguishable from those undergoing open operation at our institution.
Asymptomatic patients
Robotic and other less invasive means of performing MV repair, when performed with 99% certainty and <0.3% risk, hold specific relevance for asymptomatic patients with severe degenerative mitral regurgitation (MR). With improved understanding of the adverse natural history of patients undergoing delayed operations for severe MR, referral patterns for MV repair have changed over the past decade, from symptomatic patients often in late stages of the disease state with enlarged left ventricle (LV) size and diminished ejection fraction (EF), to young healthy asymptomatic patients found to have significant MR and normal LV function (EF >60%, LV systolic dimension <40 mm) on echocardiographic examination. The latest American College of Cardiology/American Heart Association (ACC/AHA) heart valve guidelines classify patients with chronic primary MR into four categories: Stage A (those with minimal disease), Stage B (those with progressive disease), Stage C (asymptomatic patients with severe disease), and Stage D (severe symptomatic disease). The asymptomatic group is divided into stage C1 for those without LV dysfunction [left ventricular ejection fraction (LVEF) >60% or left ventricular end systolic diameter (LVESD) <40 mm] and stage C2, those with early evidence of a failing LV (LVEF <60% or LVESD >40 mm). There is general acceptance of the need for surgery for stage D and C2 patients (class of recommendation 1, “must do”) (18).
With evidence now mounting regarding the advantages of prompt surgical correction of degenerative MR in asymptomatic patients with regurgitant volume >60 cc/beat, effective regurgitant orifice area >0.4 cm2 and preserved LV size/function (stage C1), resulting in significant survival benefit and lowered risk of heart failure, it is now imperative that these patients are fully informed regarding the benefits and risks of early referral for surgery versus watchful waiting (19-21). The newest ACC/AHA guidelines provide a class IIa (reasonable to intervene) recommendation to offer MV repair for this group of patients (when likelihood of repair is >95% and risk <1%) (18) and state that waiting for the patient to become symptomatic exposes the patient to the risks of sudden death, heart failure and poorer survival with sub-optimal outcomes of future interventions (22-25).
How then can the advantages of early intervention for asymptomatic individuals with severe MR and “normal” LV size/function (stage C1) become more widely accepted by both the referring physician and patient? With minimally invasive methods rapidly achieving critical technical goals, including the restoration of MV anatomy and near complete elimination of MR, it is expected that these procedures will provide benefits similar to those evident in the robust outcomes data associated with open repair, such as reverse LV remodeling, improved EF, protection from sudden death and normalization of survival. Additional benefits, including a minimal scar with prompt return to normal daily activities, have been noted by patients and providers, thus leading to the establishment of robot-assisted MV repair as a new standard against which emerging percutaneous technologies must compete at several major heart valve centers of excellence.
Looking towards the future: the road ahead
Robotic MV repair has changed the landscape of heart valve therapy by presenting an option which facilitates early referral and patient acceptance in those with severe MR. Although robotic programs may be challenging to establish given the infrastructure and human resource investment, they can deliver superb safety, efficacy and patient satisfaction. Percutaneous transcatheter techniques will continue to progress and improve; however, early results in degenerative disease subsets are currently sub-optimal, and thus unsuitable for the majority of such patients who are very low risk candidates for minimally invasive or robotic MV repair (risk <0.9%) (26). Emerging minimally invasive and percutaneous technologies will be closely watched by structural heart specialists around the globe and rigorously compared to well-established open surgical techniques that have traditionally delivered high quality, safe and durable outcomes capable of restoring normal life expectancy in otherwise healthy patients with severe degenerative MR (27,28). We also predict that the gap between less invasive surgical and percutaneous techniques will narrow with time. Robotic technology and instrumentation is also still evolving, and further advancements can be expected in terms of smaller and more versatile equipment, better quality optics and perhaps, inclusion of haptic feedback. Next generation robotic systems that facilitate safe learning, improved ergonomics, enhanced portability and systematic cost reduction are desired deliverables from present and future commercial vendors.
At present, only several large robotic MV repair practices exist in the world (11,12,15,16). Outcomes at these select centers are comparable if not better than results of open techniques from multicenter registry experiences, with very high (95-99%) repair rates and very low (<0.5%) mortality. Robotic MV repair has advanced degenerative MV disease therapy, and potentially its greatest value is in facilitating earlier referral and patient acceptance of a safe and effective MR elimination when class IIa indications for intervention are met.
Acknowledgements
We thank the Society of Thoracic Surgery for the providing the minor data request.
Disclosure: The authors declare no conflict of interest.
References
- McGoon DC. Repair of mitral insufficiency due to ruptured chordae tendineae. J Thorac Cardiovasc Surg 1960;39:357-62.
- Suri RM, Orszulak TA. Triangular resection for repair of mitral regurgitation due to degenerative disease. Operative Techniques in Thoracic and Cardiovascular Surgery [Internet]. 2005 Sep [cited 2014 Nov 3];10:194-9. Available online: http://www.scopus.com/record/display.url?eid=2-s2.0-30344467230&origin=inward&txGid=F13177F66D0AB13A14D49BF67565FF9F.kqQeWtawXauCyC8ghhRGJg%3a2
- Suri RM, Burkhart HM, Schaff HV. A novel method of leaflet reconstruction after triangular resection for posterior mitral valve prolapse. Ann Thorac Surg 2010;89:e53-6. [PubMed]
- Suri RM, Schaff HV, Dearani JA, et al. Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann Thorac Surg 2006;82:819-26. [PubMed]
- Carpentier A. Cardiac valve surgery--the "French correction". J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed]
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [PubMed]
- Chitwood WR Jr, Nifong LW, Elbeery JE, et al. Robotic mitral valve repair: trapezoidal resection and prosthetic annuloplasty with the da vinci surgical system. J Thorac Cardiovasc Surg 2000;120:1171-2. [PubMed]
- Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [PubMed]
- Chitwood WR Jr. Current status of endoscopic and robotic mitral valve surgery. Ann Thorac Surg. 2005;79:S2248-53. [PubMed]
- Chitwood WR Jr, Rodriguez E, Chu MW, et al. Robotic mitral valve repairs in 300 patients: a single-center experience. J Thorac Cardiovasc Surg 2008;136:436-41. [PubMed]
- Suri RM, Burkhart HM, Rehfeldt KH, et al. Robotic mitral valve repair for all categories of leaflet prolapse: improving patient appeal and advancing standard of care. Mayo Clin Proc 2011;86:838-44. [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 1410.e1; discussion 1408-10.
- Suri RM, Burkhart HM. Optimizing outcomes of robotic mitral valve repair for all prolapse anatomy: the Suri-Burkhart technique. Ann Cardiothorac Surg 2013;2:841-5. [PubMed]
- Ramzy D, Trento A, Cheng W, et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228-35. [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Suri RM, Thompson JE, Burkhart HM, et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin Proc 2013;88:1075-84. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-e132. [PubMed]
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [PubMed]
- Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs. watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609-16. [PubMed]
- Kang DH, Park SJ, Sun BJ, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol 2014;63:2398-407. [PubMed]
- Rosenhek R. Watchful waiting for severe mitral regurgitation. Semin Thorac Cardiovasc Surg 2011;23:203-8. [PubMed]
- Ghoreishi M, Evans CF, DeFilippi CR, et al. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg 2011;142:1439-52. [PubMed]
- Ngaage DL, Schaff HV, Mullany CJ, et al. Influence of preoperative atrial fibrillation on late results of mitral repair: is concomitant ablation justified? Ann Thorac Surg 2007;84:434-42; discussion 442-3. [PubMed]
- Tribouilloy CM, Enriquez-Sarano M, Schaff HV, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation 1999;99:400-5. [PubMed]
- Chatterjee S, Rankin JS, Gammie JS, et al. Isolated mitral valve surgery risk in 77,836 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg 2013;96:1587-94; discussion 1594-5. [PubMed]
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [PubMed]
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [PubMed]





