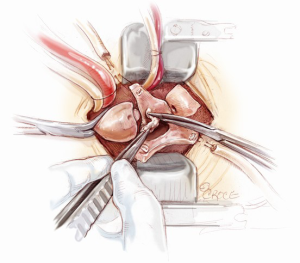
Mini-Bentall procedure
Introduction
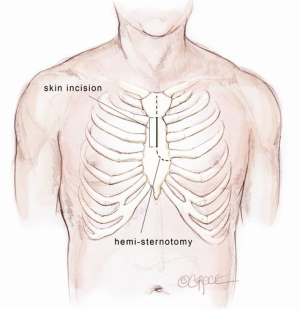
The Bentall procedure is a cardiac surgical operation involving composite graft replacement of the aortic valve, aortic root and ascending aorta, with re-implantation of the coronary arteries into the graft. This technique was first described by Hugh Bentall and Antony De Bono in 1968 (1), using a median sternotomy approach. To minimize surgical trauma and enhance patient recovery, cardiac surgeons are increasingly performing aortic valve replacement via upper hemi-sternotomy or right mini-thoracotomy incisions (2-5). The Mini-Bentall procedure consists of an aortic root and ascending aortic replacement with re-implantation of coronary buttons, performed via an upper hemi-sternotomy. The skin incision extends from the angle of Louis to the third intercostal space, usually measuring 5-7 cm in length, depending on the body size of the patient. Through this incision, it is possible to perform isolated aortic root surgery or in conjunction with hemi-arch replacement. The present article describes the technical details on how I perform a Mini-Bentall procedure and hemi-arch replacement.
Methods
Computed tomography
A careful review of three-dimensional reconstructed images of the thoracic aorta in relation to the sternum and the rib cage is necessary. This will greatly facilitate the planning for the upper hemi-sternotomy. The upper hemi-sternotomy should be terminated at one intercostal space above the plane of the aortic annulus, usually the 4th (sometimes the 3rd) intercostal space. The degree of aortic wall calcification and evidence of atheromatous disease are evaluated in both contrast and non-contrast phases. A decision regarding the most appropriate cannulation strategy is sought with the aim to minimize potential embolic risks.
Preparation
Following induction of anaesthesia, a radial arterial pressure monitor line, central venous line, pulmonary arterial sheath/catheter and urinary catheter are inserted. The patient is placed in the supine position. The body surface anatomy is clearly marked with a permanent marking pen depicting the positions of supra-sternal notch, sterno-manubrial junction, 2nd to 4th intercostal spaces, the inferior extent of xiphoid cartilage and bilateral femoral arteries. The body is painted with an antiseptic solution. Sterile drapes are placed, exposing the precordium laterally to the mid-clavicular lines and bilateral groin regions.
Skin incision
A midline skin incision is performed from the manubrio-sternal junction to the level of the third intercostal space. The incision is usually 5-7 cm in length depending on the body size of the patient. It is developed through the subcutaneous fat onto the body of the sternum using a diathermy. A cutaneous flap is developed with a diathermy hand-piece along the prepectoral fascia. This flap is extended superiorly above the supra-sternal notch and laterally to the limits of the sternum. A Kocker retractor is used to elevate the skin flap superiorly to expose the bridging vein over the manubrium and inferiorly to the 4th intercostal space. The bridging vein is clipped and cut. A 14 Fr Jackson Pratt drain (Cardinal Health, McGaw Park, IL, USA) is inserted through the skin at the level of 4th parasternal space on the left side and positioned in the subcutaneous space. This is used for CO2 inflow during the case to prevent air embolism and as a subcutaneous drain at the completion of the operation.
Mini-sternotomy
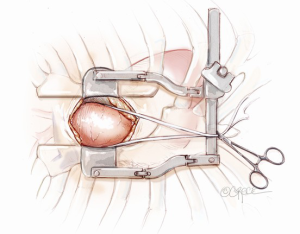
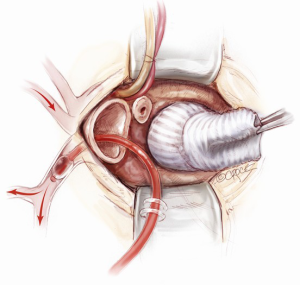
A mini-sternotomy is performed using a hand-held electrical saw from the superior extent of the manubrium. The division is terminated either to the left or right para-sternal space. In this illustrated article, a left-sided, reversed “J” hemi-sternotomy is demonstrated (Figure 1). Hemostasis is achieved by applying a small amount of wax to the bone marrow. The thymic fat pad is completely mobilized to the innominate vein superiorly. The pericardium is opened longitudinally to the pericardial reflection superiorly and the level of 4th intercostal space inferiorly. Three pericardial traction sutures are placed on each side; hitched up and tied securely to the edges of the skin incision. A minimally invasive sternal retractor is placed over the pericardial edges and opened up gradually. In doing so, the sternum is spread open, together with the pericardium, which anteriorizes the ascending aorta. A Semb clamp is passed around the ascending aorta by bluntly developing a plane between the mid-ascending aorta and the right pulmonary artery posteriorly and pulmonary artery trunk medially. The mid-ascending aorta is slung with a Nylon tape (Johnson & Johnson Intl, St-Stevens-Woluwe, Belgium). Downward traction on the Nylon tape provides additional exposure of distal ascending aorta to facilitate aortic cannulation. The access would adequately expose the aorta from the level of sinotubular junction to that of the distal ascending aorta (Figure 2).
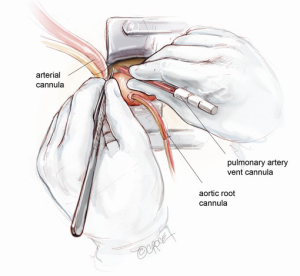
Cannulation strategy
A vacuum-assist device is built in the cardiopulmonary bypass (CBP) circuit to maximize venous drainage. Full systemic heparinization is achieved with activated clotting time (ACT) greater than 450 seconds. Peripheral venous cannulation is established first, using a Seldinger technique. After puncture of a femoral vein, a guide wire is passed up to the superior vena cava. The position of the wire is confirmed with transesophageal echocardiography (TEE) through a bicaval view. The femoral vein puncture site is then progressively dilated. A 23 or 25 Fr multi-stage venous cannula (Maquet Getinge Group, Rastatt, Germany) is introduced. The pointy-tipped insert is not advanced further once it enters the right atrium. Only the venous cannula itself is now advanced forward over the insert, strictly under the TEE guidance. It is essential that the cannula tip be placed in the superior vena cava to ensure satisfactory bicaval venous return. The venous cannula is subsequently connected to the CPB circuit.
Arterial cannulation can be established either via distal ascending aorta or femoral artery. In this illustrated article, a distal ascending aortic cannulation is depicted. The Nylon tape slung around the mid-ascending aorta is retracted inferiorly. The superior edge of the skin incision is elevated cephalad using a Kocker tractor. Two 2-O Ti-Cron purstrings for the aortic cannula are placed in the distal ascending aorta at the level of pericardial reflection. The aorta is carefully cannulated with an Elongated One-Piece Arterial (EOPA) cannula (Medtronic Inc, Minneapolis, MN, USA), which is secured in position and connected to the CPB circuit.
A 16 Fr DLP pulmonary artery vent cannula (Medtronic Inc, Minneapolis, MN, USA) is inserted in the main pulmonary trunk (Figure 3). Once CPB is established, the heart is off-loaded. Systemic temperature is maintained at 32 degrees centigrade for aortic root replacement or 25 degrees if hemi-arch replacement is anticipated. In the latter, splitting the arterial line is necessary beforehand to provide additional cerebral perfusion.
Aneurysm resection
Under a low-flow condition, an atraumatic aortic cross-clamp is applied across the distal ascending aorta. Diastolic arrest is achieved with antegrade cardioplegia delivered via a DLP aortic root cannula (Medtronic Inc, Minneapolis, MN, USA) or direct coronary ostial balloon tip cannula (Maquet Getinge Group, Rastatt, Germany) in the presence of aortic regurgitation. Either cold-blood cardioplegia solution or Custodial cardioplegia solution is suitable.
After aortotomy, the blood in the aortic root is salvaged. The aneurysmal segment of the ascending aorta is resected, leaving 1 cm cuff of aortic tissue proximal to the cross-clamp and 1 cm above the sinotubular junction (Figure 4). The inside of the aortic root is assessed and the positions of the coronary arteries are visualized. The aortic root is carefully mobilized circumferentially. First, the non-coronary sinus is resected, leaving an 8 mm rim of aortic wall just above the non-coronary annulus. Then, the right coronary button flap is prepared. Two vertical incisions are made from the sinotubular junction down along both sides of the right coronary ostium and connected inferiorly (Figure 5). A 4-0 polypropylene suture is passed through the top end of the right coronary button flap to provide gentle traction when required. Similarly, the left coronary button flap is also fashioned. Often only limited mobilization of the coronary buttons from the surrounding connective tissue is required.
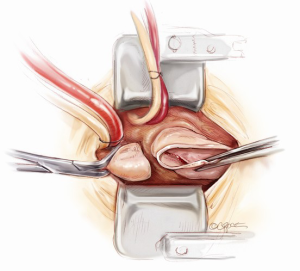
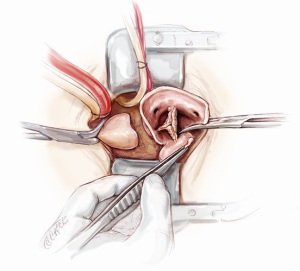
Aortic root exposition
In order to provide an excellent exposure, the aortic root is brought in a cephalad direction. This is achieved by putting the first pledgeted 2-0 Ti-Cron horizontal mattress suture above the non-/left-coronary commissure. The needles are then passed through the edge of the skin incision on the right side, at the 10 o’clock position. The traction suture is snared and clipped. The needles of the next pledgeted 2-0 Ti-Cron suture are passed through just above the left-/right-coronary commissure and the skin edge on the left side at the 2 o’clock position. The third pledgeted 2-0 Ti-Cron traction suture is used to elevate the right-/non-coronary commissure and attached to the skin edge on the right side at the 7 o’clock position. These three commissural traction sutures are hitched up and snared in position. This simple manoeuvre provides an excellent exposure of the aortic valve for the minimal access surgical approach (Figure 6).
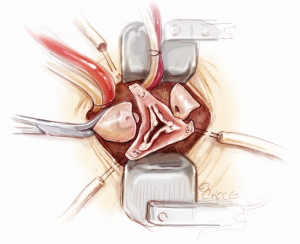
Aortic root implantation
The aortic leaflets are resected and the aortic annulus is decalcified (Figure 7). 2-0 Ethibond Excel annular sutures with or without pledgets are used. Horizontal mattress sutures are placed neatly below the aortic annulus. Pledgets are used to reduce the tension created by the sutures when the valve conduit is tied down, especially at the nadirs. The placement of these sub-annular sutures needs to be precise, both in terms of the spacing and whether or not the needle is passed through the annulus at a perpendicular angle (Figure 8). The sutures are evenly distributed.
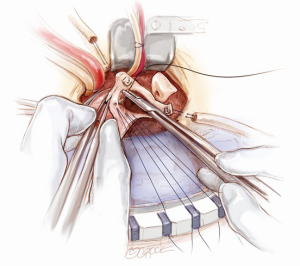
The annulus is sized for its intra-annular and supra-annular dimensions. An appropriate valve conduit is selected. It is advisable not to oversize the valve. The annular sutures are passed through the sewing cuff of the prosthesis. Once all the sutures are passed through the sewing cuff, they are clipped and cut. The valve conduit is parachuted down by gently pulling the sutures vertically upwards with one hand and firmly pushing the valve conduit down onto the annulus with the other hand (Figure 9). Before tying each suture, it is important to check that there are no redundant loops of the sutures below the sewing cuff. The sutures are tied and cut one by one, starting from the three sutures at the nadirs of the annulus to ensure that the prosthesis is seated properly to the lowest points of the annulus. Then, the remaining sutures are tied and cut around the sewing cuff.
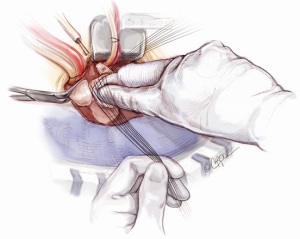
Coronary button re-implantation
The left coronary artery button is rested in its anatomical position. The appropriate site on the valsalva graft (Vascutek Ltd, Renfrewshire, Scotland) for left coronary button re-implantation is determined with the heart fully loaded, so that there is no tension or rotation of the left coronary button anastomosis. Once the position of the anastomosis is marked, the heart is off-loaded. A Bovie electrocautery (Bovie Medical Corporation, Clearwater, FL, USA) is used to create a circular hole for receiving the left coronary button. The coronary button is trimmed, left with a 3 mm circumferential cuff and re-implanted using a 5-0 running polypropylene suture. The cuff of the coronary button needs to be attached snuggly to the outside of valsalva graft (Figure 10). In a similar fashion, the right coronary button is prepared and re-implanted (Figure 11). It is imperative to ensure that a full thickness bite with each stitch is achieved. Failing to do so may cause bleeding in a very difficult site to access once the cross-clamp is taken off. The aortic root is pressurized and the anastomoses are tested by delivering a full dose of antegrade cardioplegia. Systemic rewarming is subsequently initiated.
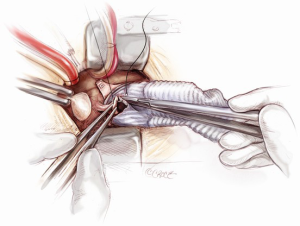
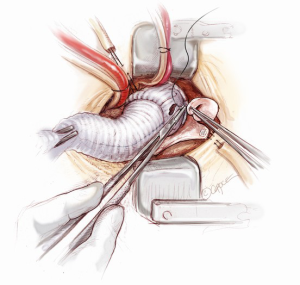
Distal anastomosis
The distal aortic anastomosis is reconstructed with the aortic cross-clamp on. The length of the graft is determined by filling the heart up and pulling the graft upwards on a stretch to meet distal ascending aorta. The graft is trimmed and anastomosed to the distal ascending aortic cuff by using a 3-0 running polypropylene suture. The anastomosis is started from the point furthest away from the operating surgeon. The posterior wall is completed first from 4 o’clock to 11 o’clock position on the aorta (Figure 12). A nerve hook is used to tighten the posterior wall suture progressively towards the operating surgeon. The anterior half of the anastomosis is completed by picking up the other end of the suture and sewing towards the operator. An aortic root vent is inserted before the two ends of the suture are tied. The table is put in a Trendelenburg position, the heart is filled and lungs are inflated, the arterial flow is turned down, aortic cross-clamp is release and the graft is de-aired from the aortic root vent.
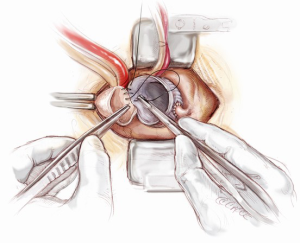
Hemi-arch replacement
If the distal ascending and/or proximal arch are aneurysmal, a hemi-arch replacement with an open distal anastomosis is performed. Two cerebral perfusion cannulae are prepared at the beginning of the case in readiness for antegrade cerebral perfusion. Systemic cooling down to 25 degrees is initiated. As soon as the systemic temperature reaches 25 degrees, attention is then turned to the distal hemi-arch replacement. The head is packed in ice, the antegrade flow is ceased, the aortic cannula is clamped, the aortic cross clamp is removed and the patient’s blood is drained into the reservoir. The mid to distal ascending aorta is resected completely, together with the cannulation site. The under surface of the proximal aortic arch is beveled. The inside of the aortic arch and the origins of epi-aortic vessels are inspected for any evidence of atheromatous disease. Selective antegrade cerebral perfusion is achieved by cannulating the innominate artery with or without left common carotid artery (Figure 13). The open distal anastomosis is performed using a separate Ante-Flo graft with a single side arm (Vascutek Ltd, Renfrewshire, Scotland). A continuous 3-0 running polypropylene suture is used, starting from the point furthest away from the operating surgeon and completing the posterior aortic wall first (Figure 14). A nerve hook is used to tighten the suture along the posterior wall. The anterior half of the anastomosis is completed by picking up the other end of the suture and sewing towards the operator. This distal anastomosis is reinforced with pledgeted 4-0 prolene sutures where necessary to ensure an absolute hemostasis. After completion of the open distal anastomosis, antegrade systemic perfusion is resumed via the side-arm perfusion limb of the Ante-Flo graft. The patient is rewarmed towards 37 degrees centigrade.
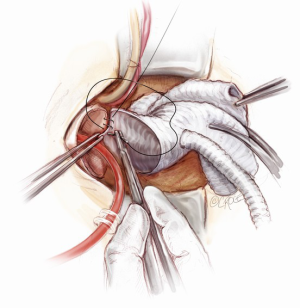
Once the root procedure is completed, the proximal valve conduit is trimmed just above the valsalva portion of the graft. The distal Ante-Flo graft is put under a stretch and cut to an appropriate length. A graft-to-graft anastomosis is performed using a continuous 3-0 running prolene suture (Figure 15). The spacing between the adjacent stitches needs to be narrow and precise when doing a graft-to-graft anastomosis, usually a couple of millimeters apart. Finally, the aortic root vent is inserted, the aortic cross clamp is slowly released and a 21-gauge needle is used to de-air the graft. In order to ensure an absolute hemostasis, pledgeted 4-0 prolene sutures are applied to reinforce the proximal anastomosis when necessary.
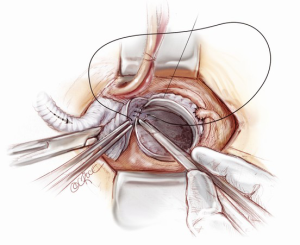
Completion
A bi-polar temporary pacing wire is inserted in the epicardium over the right ventricular outflow tract. One 28 Fr soft drain is inserted and brought out below the xiphoid cartilage. Hemostasis is carefully checked and the patient is weaned from CPB. Protamine is given to reverse the Heparin effect. Topical Floseal Hemostatatic Matrix (Baxter Healthcare, Zurich, Switzerland) is applied around the anastomotic sites. The surgical site is packed with small gauze sponges for a period of 10-minute hemostatic pause. Once the hemostasis is deemed satisfactory, four stainless steel wires are used to approximate the sternum. No. 1 Vicryl suture is used to close the fascia and the subcutaneous fat. The skin is closed with a 5-0 Monocryl subcuticular suture. This completes the Mini-Bentall procedure.
Discussion
An important goal in modern cardiovascular and thoracic surgery is reducing surgical trauma to achieve faster recovery for our patients. The benefits of minimally invasive surgery are evident (4,6-8). More surgeons are comfortable with aortic valve replacement via upper hemi-sternotomy or right mini-thoracotomy, and thus naturally there is a growing interest in performing aortic surgery via a minimal access incision. The present illustrated article described the technical details of Mini-Bentall procedure and hemi-arch replacement for selected patients with aortic root and ascending aortic aneurysms.
The mini-sternotomy is performed using a hand-held electrical saw from the superior extent of the manubrium. The division is terminated either to the left or right para-sternal space. One advantage to the right (a “J” mini-sternotomy) is avoiding any potential injury to the left internal mammary artery, should it be used for coronary bypass surgery in the future. In addition, access to the right superior pulmonary vein for venting is made easier. The main advantage of doing a mini-sternotomy to the left (a reverse “J”) is increasing the exposure of proximal arch, especially if a concomitant hemi-arch replacement is anticipated. Under this circumstance, a pulmonary artery vent is used instead of right superior pulmonary vein vent. It should be cautioned that when inserting a pulmonary artery vent, the heart needs to be kept full or even before going on CPB. This will avoid injury to the posterior wall of the pulmonary trunk with the tip of the pulmonary artery cannula.
Arterial cannulation can be established either via distal ascending aorta or femoral artery. It is preferable to have a central arterial cannulation whenever possible to provide adequate antegrade systemic perfusion and avoid potential retrograde embolization and vascular complications that may be associated with peripheral cannulation (9). Should femoral arterial cannulation be used, a 2.5 cm oblique incision is performed in the groin, exposing the anterior aspect of the common femoral artery. A 5-0 polypropylene purstring suture is placed in the anterior aspect of the common femoral artery. The artery is cannulated using a Seldinger technique. A guide wire is passed up in the descending thoracic aorta. Its presence within the descending aorta is confirmed with TEE. After progressive manual dilatation of the femoral artery puncture site, a wire re-enforced femoral arterial cannula should be inserted without any resistance.
In order to provide adequate exposure and surgical accessibility, it is important to anteriorize the aortic root, as well as bring the aortic annulus cephalad. This is achieved by placing three pledgeted 2-0 Ti-Cron horizontal mattress sutures above the commissures and hitching them up to the skin edges. This simple manoeuvre provides an excellent exposure of the aortic valve for the minimal access surgical approach. Even though the mini-sternotomy terminating at the level of sinotubular junction, this manoeuvre could bring the aortic annulus forward in the cephalad direction by 2 to 3 cm.
Composite graft replacement of the ascending aorta and aortic valve was first introduced by Bentall and De Bono in 1968 (1). According to this technique, the aortic tissue surrounding the coronary ostia is directly sutured to the openings in the composite graft. These anastomoses were all made within the ascending aorta, and then the aortic wall is tightly wrapped over the conduit. This technique has been known as the wrap/inclusion technique (1). Coronary artery dehiscence and coronary false aneurysms may result from tension created by bleeding into the space between the graft and the wrap (10). A few technical modifications have been implemented, including the use of a reinforcement suture joining the cut edge of the aortic wall and the prosthetic sewing ring (11). This technique described two separate proximal suture lines, an interrupted one between the most proximal part of the valve sewing ring and the aortic valve annulus, and a continuous one between the more distal portion of the sewing ring and the cut edge of the proximal aorta. In a Mini-Bentall procedure, absolute hemostasis must be achieved. If there are any concerns regarding the hemostasis along the annulus (due to suture spacing or severely calcified annulus), a second ‘hemostatic’ layer is achieved by using a continuous 4-0 running polypropylene suture that incorporates the 8 mm remnant of the aortic wall and the sewing cuff of the valve conduit. This technique works well for a mechanical valve conduit (St. Jude Medical, St. Paul, MN, USA), as the sewing cuff is big enough to accommodate two rolls of anastomoses. However, when a bioprosthetic valve is used inside of a valsalva graft, whereby the sewing ring of the tissue valve is narrow and the second ‘hemostatic’ layer is difficult to achieve, a ‘French Cuff’ technique is used (12).
In the strategy presented, the fundamental principles of a traditional aortic root replacement are respected and it cannot be emphasized enough that a meticulous surgical technique to ensure absolute hemostasis is utmost important in minimally invasive surgery. This results in a complete aortic repair via a minimal access incision and successful treatment in selected patients with aortic root and/or ascending aortic aneurysm.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [PubMed]
- Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg 1996;62:1542-4. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [PubMed]
- Phan K, Xie A, Di Eusanio M, et al. A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg 2014;98:1499-511. [PubMed]
- Phan K, Xie A, Tsai YC, et al. Ministernotomy or minithoracotomy for minimally invasive aortic valve replacement: a Bayesian network meta-analysis. Ann Cardiothorac Surg 2015;4:3-14. [PubMed]
- Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. [PubMed]
- Phan K, Zhou JJ, Niranjan N, et al. Minimally invasive reoperative aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:15-25. [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11.
- Benedetto U, Raja SG, Amrani M, et al. The impact of arterial cannulation strategy on operative outcomes in aortic surgery: evidence from a comprehensive meta-analysis of comparative studies on 4476 patients. J Thorac Cardiovasc Surg 2014;148:2936-43.e1-4.
- Nezic D, Cirkovic M, Knezevic A, et al. Modified Bentall procedure - 'a collar technique' to control bleeding from coronary ostia anastomoses. Interact Cardiovasc Thorac Surg 2008;7:709-11. [PubMed]
- Copeland JG 3rd, Rosado LJ, Snyder SL. New technique for improving hemostasis in aortic root replacement with composite graft. Ann Thorac Surg 1993;55:1027-9. [PubMed]
- Yan TD. Modified Bentall Procedure - the ‘French Cuff’ technique. (In Press).