In the era of the valve-in-valve: is transcatheter aortic valve implantation (TAVI) in sutureless valves feasible?
Introduction
While conventional aortic valve replacement (c-AVR) represents the gold-standard treatment in low risk patients with severe aortic valve stenosis, sutureless aortic valve replacement (s-AVR), and transcatheter aortic valve implantation (TAVI) are increasingly offered as minimally invasive alternatives to symptomatic patients with a higher surgical risk profile (1). Similarly, transcatheter aortic valve-in-valve implantation (A-ViV) is emerging as a valuable procedure in patients with dysfunctioning biological aortic valves who are deemed inoperable with conventional surgery (2,3). While successful A-ViV procedures have been reported in patients with sutured stented or stentless aortic valve prostheses, there have been no reports available to date that demonstrate the feasibility of A-ViV procedures for sutureless aortic valve prostheses. Here we present the first-in-man case of TAVI with a balloon expandable device in a sutureless self-expandable valve.
Clinical vignette
A frail 80-year-old woman with worsening dyspnea upon exertion due to severe aortic stenosis was recently admitted to our hospital. The patient’s medical history revealed a previous percutaneous coronary intervention (PCI) with bare metal stent implantation for a double vessel coronary artery disease. Main co-morbidities and risk factors included obesity (BMI: 30.8 kg/m2), hypertension, hypercholesterolemia, moderate pulmonary hypertension, and paroxysmal atrial fibrillation. Echocardiography showed a severe aortic stenosis (max/mean aortic valve gradient 93/51 mmHg, aortic valve area 0.5 cm2) with preserved left ventricular systolic function and a severely calcified mitral valve with moderate regurgitation. The local heart team discussed the case, and the decision to perform s-AVR by a minimally invasive approach was made. Pre-operative coronary angiography ruled out a significant progression of the coronary disease and demonstrated good results from the prior PCI.
The surgical procedure was performed under general anesthesia. The heart was exposed through an upper J mini-sternotomy at the 4th intercostal space and cardiopulmonary bypass was established with standard central cannulation. The aortic valve cusps were excised and an accurate decalcification of the aortic annulus was performed. A PERCEVAL S valve (Sorin Group, Saluggia, Italy) was selected for s-AVR. Sizing (size M) and implantation were performed as recommended. The correct valve positioning was visually checked. No complications occurred during the intervention and no intraoperative manoeuvres such as cardiac massage or rough aortic manipulation were performed. Intraoperative trans-esophageal echocardiography (TEE) confirmed correct valve positioning and ruled out any valvular leakage or significant gradient across the valve.
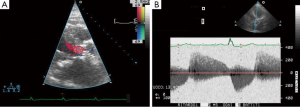
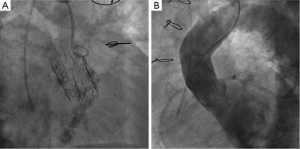
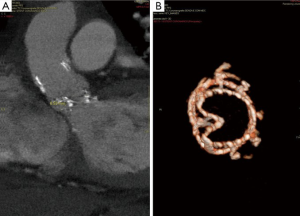
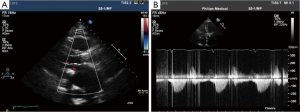
Post-operatively, no major complications occurred with the exception of moderate renal insufficiency (creatinine peak: 2.2 mg/d, pre-operative value: 0.9 mg/dL). On post-operative day 5, a trans-thoracic echocardiography (TTE) revealed a severe aortic valvular regurgitation, which was unexpected given the intraoperative course, but consistent with the increased patient requirement for diuretics. Peak and mean trans-valvular gradients were 36 and 19 mmHg, respectively (Figure 1A,B). The severity of the regurgitation was confirmed by angiography (Figure 2A,B), and computed tomography (CT) scan clearly showed that the inflow stent of PERCEVAL S valve was partially collapsed at the non-coronary sinus, the latter appearing less developed than the coronary ones (Figure 3A,B). The annulus diameter measured 21.5 mm, confirming a correct valve sizing; no prosthesis displacement was found. Given the high-risk profile of the patient, the same heart team decided to manage this complication using a percutaneous approach. Under mild sedation, via a transfemoral approach, percutaneous ballooning of the sutureless prosthesis was performed. However, while the Perceval S valve expanded well during the balloon inflation, an immediate recoil with inward protrusion of part of the stent frame was evident after balloon deflation. Subsequently, using rapid pacing and TEE guidance, a Edwards Sapien XT 23 mm (Edwards SAPIEN XT, Edwards Lifesciences Inc, Irvine, CA, USA) was successfully deployed through the same vascular access with excellent angiographic results (Videos 1,2). The TAVI sizing was based on the internal diameter of the Perceval S prosthesis, which measures between 19.5 and 21 mm, varying based on the actual final expansion; these values are compatible with a 23 mm Sapien XT. The post-procedural course was uneventful; renal function improved and TTE at discharge showed no significant leaks and gradients across the A-ViV (Figure 4).
Discussion
S-AVR has emerged as an innovative alternative for treatment of aortic stenosis. By avoiding the placement of sutures, this approach aims to improve surgical outcomes by facilitating less traumatic minimally invasive approaches and reducing cross-clamp and cardiopulmonary bypass duration (4). As for TAVI, however, the absence of sutures may have detrimental effects after s-AVR interventions, namely paravalvular leakages and valve dislocation. Paravalvular leakage most frequently results from incorrect positioning of the valve due to an incomplete decalcification of the annulus or due to wrong sizing. When found intra-operatively, at visual inspection or intraoperative TEE, the surgeon can easily remove the prosthesis and proceed to a new implant. Some authors, however, have described paravalvular leakages found post-operatively. If these are less than moderate, conservative management with clinical and echocardiographic follow-up is usually performed, otherwise reoperation is the main option.
In our case, the cause of the stent infolding remains unknown. However, despite a standard sizing and a well-compliant decalcified aortic annulus, based both on CT imaging and on instantaneous return to infolding position of the Perceval S valve after balloon deflation at secondary procedure, our prosthesis acted as if it was oversized. Although we acknowledge it is a mere speculation, we suppose an asymmetric annulus eventually led to the valve infolding at the level of the non-coronary sinus.
Options for treatment in our case included: balloon dilatation, A-ViV implantation, and standard reoperative AVR. The high-risk profile of our patient led us to choose the least traumatic option: attempting balloon dilatation of the sutureless prosthesis, followed by A-ViV implantation using a Edwards Sapien XT. In fact, the structural deterioration occurring in our case was similar to the so called ‘stent creep’ described for older surgical bio-prostheses, defined as a permanent inward deflection of the stent posts. Successful treatment of this complication with transcatheter A-ViV has been recently reported (5). In our patient, the radial force of Edward Sapien XT enabled full expansion of the sutureless infolded valve with resolution of the valvular leak. Valve sizing was very important in our case, as in all TAVI procedures. It is worth highlighting that correct valve sizing for ViV procedures requires the precise knowledge of the actual internal diameter of the prosthesis. In fact, different types of bio-prostheses with the same nominal size may have extremely different inner and outer diameters based on the engineering of the device.
In this report we documented the feasibility of an A-ViV intervention using a balloon-expandable valve in a leaking self-expanding sutureless one. We believe this may represent a valuable option of treatment in selected high-risk patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mylotte D, Osnabrugge RL, Windecker S, et al. Transcatheter aortic valve replacement in Europe: adoption trends and factors influencing device utilization. J Am Coll Cardiol 2013;62:210-9. [PubMed]
- Dvir D, Webb J, Brecker S, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 2012;126:2335-44. [PubMed]
- Bapat V, Attia R, Redwood S, et al. Use of transcatheter heart valves for a valve-in-valve implantation in patients with degenerated aortic bioprosthesis: technical considerations and results. J Thorac Cardiovasc Surg 2012;144:1372-9; discussion 1379-80. [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11.
- Attia R, Papalexopoulou N, Hancock J, et al. Successful treatment of failing biological prosthesis due to ‘stent creep’ with Valve-in-Valve Transcatheter Aortic Valve Implantation. Catheter Cardiovasc Interv 2014. [Epub ahead of print]. [PubMed]





