Quantitation of mitral regurgitation after percutaneous MitraClip repair: comparison of Doppler echocardiography and cardiac magnetic resonance imaging
Introduction
Patients with severe mitral regurgitation (MR) have a poor prognosis, particularly when surgical intervention is contraindicated or high risk (1-3). Percutaneous valve interventions for severe, symptomatic MR are emerging treatments for patients with high surgical risk. Percutaneous mitral valve intervention has been advocated for selected patients in the most recent update of the European heart failure guidelines (4). The EVEREST I and II trials demonstrated the clinical utility and safety of the MitraClip device (Abbott Vascular Structural Heart, Menlo Park, California, USA), for a percutaneous edge-to-edge repair (5,6).
Whilst transthoracic echocardiographic quantification of valvular regurgitation is recommended to identify patients at risk of adverse long-term physiologic consequences, quantification of residual MR following a MitraClip is challenging due to several reasons. These include the presence of the cliplimiting visualization of the jet origin, eccentricity of the MR jets, potential multiple sites of regurgitation, the dynamic nature and altered anatomy of the MR orifice secondary to the presence of a clip after the edge-to-edge “Alfieri”-type repair (7-10). There is a need for improved quantification of residual MR after percutaneous valve intervention.
Cardiovascular magnetic resonance (CMR) provides a quantitative and reliable mechanism to assess ventricular and valvular structure and function (11-13). The MitraClip is a magnetic resonance imaging (MRI)-conditional device and can be safely imaged using CMR (14). Flow CMR imaging provides a direct measure of forward and regurgitant flow across cardiac valves (15-17), and has been demonstrated to have high accuracy and reproducibility for quantification of aortic and MR (18-24). CMR has several potential advantages compared to transthoracic echocardiography (TTE) in this regard, including improved endocardial definition, fewer geometric assumptions and reduced angle dependence as compared to Doppler echocardiography. Use of CMR has been previously reported after percutaneous mitral valve repair (14,25); however, there is no data comparing echocardiography and CMR for quantification of any residual MR (26,27). In this prospective study, quantitative Doppler echocardiography and comprehensive CMR were performed on the same day in patients after MitraClip percutaneous mitral valve repair. The aim was to assess the reproducibility of each technique in the assessment of any residual regurgitation following MitraClip percutaneous valve repair.
Inclusion criteria
Consecutive patients with severe, symptomatic MR at high risk for cardiac surgery having undergone successful MitraClip percutaneous valve intervention. Patients were enrolled during the routine 30-day post-procedure clinic visit.
Exclusion criteria
Patients with an implanted pacemaker or defibrillator, other non-MRI safe implants, severe claustrophobia, arrhythmia preventing adequate MRI gating, and/or inability to provide informed consent were excluded from the study.
Methodology
Echocardiography
TTE was performed using a commercially available ultrasound platform (Philips iE33, Best, The Netherlands) with two-dimensional (2D) imaging and Doppler data including dedicated pulsed wave (PW) and continuous wave (CW) imaging. Total stroke volume (TSV) was defined as the total volume of blood ejected by the left ventricle (LV) in systole, which included both the regurgitant volume (RVol) and the forward stroke volume (FSV) delivered to the peripheral systemic circulation (28-30).
Assessment of regurgitation
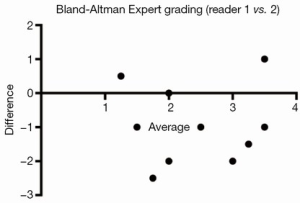
Severity of MR was initially assessed by integrated multiparametric visual evaluation in accordance with standard clinical practice (incorporating 2D, spectral and colour Doppler images; Figure 1) using an ordinal scale (grading 0-4) by two blinded expert readers with >15 years’ experience in quantitative Doppler echocardiography.
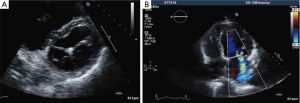
Echocardiographic quantitative assessment of regurgitant volume (RVolDoppler) and fraction (RF%Doppler) was performed by measuring the transvalvular antegrade Doppler stroke volumes (SV) at the level of the left ventricular outflow tract (LVOT), compared to the inflow SV at the mitral annulus, as recommended in the American Society of Echocardiography (ASE) guidelines (28). Proximal isovelocity surface area (PISA) method for calculation of mitral regurgitant orifice area (ROA) was also performed (28).
Cardiac magnetic resonance imaging
CMR images were acquired immediately after the TTE images on a commercially available MRI system (1.5 Tesla Magnetom AERA, Siemens, Erlangen, Germany) using a dedicated 18 channel phased array cardiac receiver coil. Cine CMR (TruFISP) images were acquired in multiple planes for left ventricular function, SV, mitral anatomy and anatomic regurgitant orifice area in the short axis view (TE 1.3 ms, TR 3 ms, flip angle 70, receiver bandwidth 914 Hz/pixel, FOV 380 mm, slice thickness 8 mm, slice gap 2 mm, in-plane spatial resolution 1.3 mm × 1.3 mm, vector ECG gating, temporal resolution ~30 ms, acceleration factor 2).
Breath-held through-plane flow CMR imaging was performed perpendicular to the aorta (using two localiser planes) at the level of the main pulmonary artery bifurcation for assessment of left ventricular SVs (TE 2.9 ms; TR 5 ms; flip angle 20; temporal resolution ~40 ms; in-plane spatial resolution 2 mm × 2 mm; slice thickness 6 mm; FOV 380 mm; VENC 110 cm/s; receiver bandwidth 491 Hz/pixel).
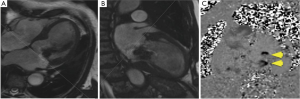
Breath-held through-plane flow CMR imaging was acquired parallel to the mitral valve annulus immediately atrial to the MitraClip device. The optimal acquisition plane was carefully planned using both vertical and horizontal long axis systolic frame cine images (Figure 2), and positioned to avoid susceptibility artefacts from the clips whilst capturing both forward and regurgitant transmitral flow.
CMR quantitation
LV volumes were measured by tracing endocardial borders of stacked short axis images obtained from Cine CMR images covering the entire LV from base to apex, using dedicated third-party software (CVi-42 release 4.1.5, Circle Cardiovascular Imaging, Calgary, Canada). Papillary muscles and trabeculations were included in the blood pool. Care was taken to contour the left ventricular endocardium using the moving cine image as a reference, particularly as the basal slice was affected by varying degrees of susceptibility artefact from the MitraClip devices.
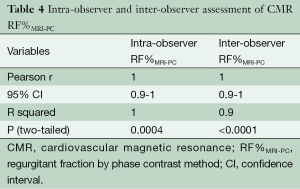
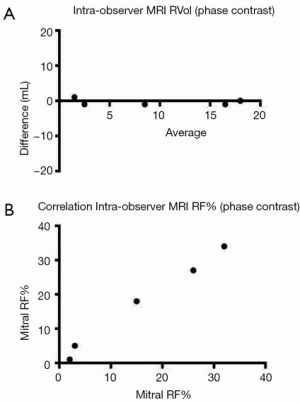
Standard SSFP imaging (TruFISP) was used for ventricular quantification, as the definition between myocardium and blood pool was not judged to be significantly compromised by susceptibility artefact, which was largely contained within the blood pool and did not affect the endocardial border in most cases. Two blinded observers performed the LV contours on SSFP imaging, using the moving cine image of each slice to determine the endocardial border. The agreement between these two observers was excellent [Pearson R-square 0.9, 95% confidence interval (CI), 0.9-1.0; P<0.0001].
Regurgitant volume and fraction were derived by two methodologies:
- “Stroke volume method” comparing left ventricular SV calculated from cine CMR short axis imaging against the forward SV in the proximal ascending aorta as assessed by flow CMR imaging [regurgitant volume (RVolMRI-SV) and regurgitant fraction (RF%MRI-SV)];
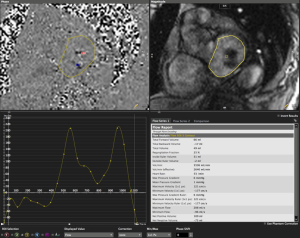
- “Phase-contrast method” using the dedicated flow CMR acquisition immediately atrial to the MitraClip device, analyzed using planimetry of the mitral inflow orifice on magnitude images (Figure 3), and computer-generated calculation of regurgitant volume (RVolMRI-PC) and regurgitant fraction (RF%MRI-PC). Severity of phase contrast MRI regurgitant fractions were categorized using previously published ranges (<15%=1 mild, 16-25%=2 moderate, 26-48%=3 moderate-severe, >48%=4 severe) (31).
Data measurements and statistical analysis
Echocardiographic multiparametric grading scale and quantitative Doppler measurements were made by two independent expert level three readers, blinded to the measurements of the other observer and to the CMR results. Similarly, CMR measurements were made by two level three blinded observers using a commercially available CMR analysis platform (CVi42, Circle, Calgary, Canada).
Baseline characteristics were reported using descriptive statistics, with means, standard deviations and ranges. Inter-observer variability for TTE and CMR was determined using both Bland-Altman (BA) method (plot of the difference of means, limits of agreement and bias), and the concordance correlation coefficient (CCC) with corresponding 95% CIs, Pearson’s statistic (P) and bias correlation coefficient (Cb). Intra-observer reproducibility was blindly performed on a subset of five studies. Statistical analysis was performed using MedCalc (version 11.0.0, MedCalc Software, Mariakerke, Belgium) and GraphPad Prism (v6 GraphPad Software, La Jolla, CA, USA).
The study was approved by the local Human Research Ethics Board (HREC/13/QPCH/67) and all patients gave written informed consent. Funding was provided by the Richard Slaughter Centre of Excellence in Cardiovascular Magnetic Resonance, the Smart Futures Fellowship Grant (Department of Science, Information Technology, Innovation and the Arts, Queensland Government), and an unrestricted educational grant from Abbott Vascular who had no involvement in the study design, analysis or writing.
Results
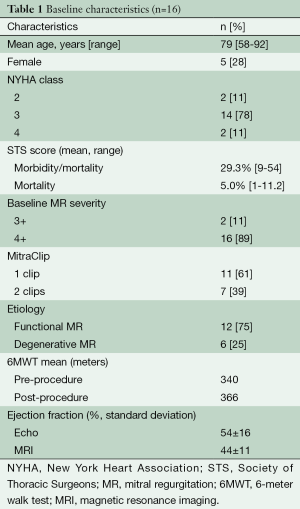
Twenty-five patients underwent successful MitraClip percutaneous mitral valve intervention for symptomatic severe MR during the study period. Nine patients were excluded from the study due to non-MRI safe devices or severe arrhythmia (atrial fibrillation preventing adequate MRI gating), leaving 16 patients to have same-day quantitative echocardiography and CMR examinations, with successful image acquisition in all patients. Baseline characteristics are presented in Table 1.
Full table
Echocardiography
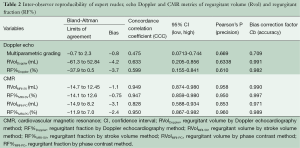
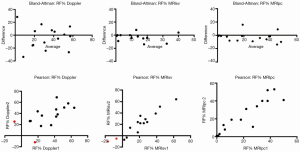
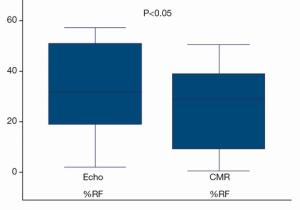
Integrated multiparametric ordinal grading between two expert readers had a CCC of 0.47, Pearson’s P of 0.67, and bias correction factor Cb of 0.70 (Table 2, Figure 4). Quantitative Doppler echocardiography had a CCC of 0.598, Pearson’s P of 0.60, bias correction factor Cb of 0.98, indicating good inter-observer agreement (Table 2). However, the BA analysis showed wide limits of agreement −37.9 to 30.5 (Figure 2) and bias −3.7% (Table 2, Figure 2). In two patients, Doppler echocardiography calculated “negative” regurgitation, despite clear presence of a regurgitant jet on colour Doppler imaging. This occurred when the calculated forward SV through the aortic valve exceeded the ventricular SV, highlighting the challenges of this method. The PISA method was technically unsuitable for mitral regurgitant quantification due to significant artefacts from the MitraClip device(s) obscuring the PISA flow convergence zone, and the presence of multiple regurgitant jets.
Full table
Cardiac magnetic resonance
The SV method of CMR (RF%MRI-SV: comparing ventricular SV to aortic forward volume) had excellent correlation between observers (R-square 0.9). However, two patients had nonsensical “negative regurgitation” due to measured aortic flow being higher than left ventricular SV. This is a limitation of the SV technique (both in Doppler echo and CMR), which employs a comparison of two different measurements, each with inherent artefacts.
The CMR phase contrast technique (RF%MRI-PC) also had excellent inter-observer correlation R-square 1.0, 95% CI, 0.9-1.0 (P<0.0001) CCC 0.95, Pearson’s precision 0.96 and bias correction factor 0.989; all superior to Doppler echocardiography (Tables 2,3). BA analysis showed narrow limits of agreement (−11.0 to 7.0) with bias of regurgitant fraction at −2.4% (Tables 2,3, Figures 5,6).

Full table
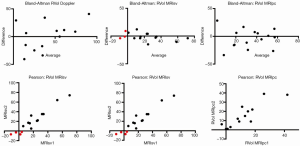
However, in one patient, CMR phase contrast imaging calculated a low regurgitant volume and fraction (11%), despite a significant regurgitant jet (grade 3+) seen on both CMR-SSFP imaging and colour Doppler echocardiography. This patient had a “swinging jet” which, during ventricular systole, changed direction from central towards the lateral wall and atrial appendage, due to systolic prolapse of the mitral valve leaflets and the MitraClip device. In this situation, the MRI-SV technique provided an adequate regurgitant volume and fraction. Thus, it appears that the two different MRI techniques (MRI-SV and MRI-PC) have differing strengths and may be used together.
Intra-observer and inter-observer reproducibility of RF%MRI-PC showed excellent correlation (Table 4) with narrow limits of agreement on BA assessment (Table 3, Figure 7).
Full table
Reclassification of regurgitation severity
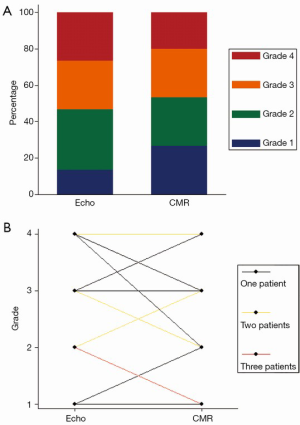
The severity grading of residual MR (grades 1-4 representing mild, moderate, moderate-severe, severe) was compared using the ASE guidelines for Doppler quantitation (29), and published ranges for CMR quantitation (31) (Figures 8,9).
Discussion
CMR had superior reproducibility compared to echocardiography for quantification of residual MR after percutaneous MitraClip repair, with lower limits of agreement and superior concordance coefficient (CCC), precision (P) and accuracy (Cb) measurements. A strength of the study is that experienced imaging cardiologists performed both the echocardiographic and CMR analyses. Despite this, only moderate agreement was present between expert readers for the visual assessment of residual MR. This underlines the need for more reproducible, quantitative methods.
Echocardiography is the most commonly used technique to assess valvular regurgitation. However it has significant limitations following percutaneous MitraClip insertion. The double-orifice mitral valve (Figure 1A) typically creates multiple, complex eccentric regurgitant jets. When combined with the artefacts from the clip, assessment of regurgitation by colour and spectral Doppler can be challenging, as illustrated by the lack of agreement between expert readers (Figure 6). The PISA method is a quantitative technique which has prognostic data for native MR (29). However, our data show that this method was not technically suitable after MitraClip repair, due to artefacts from the clips. Quantitative Doppler using comparison of SVs at the LVOT and mitral annular planes is a well-validated technique (28). However, in the present data, both the measurement of the mitral annular dimension and the pulse wave sample volume positioning were hampered by the MitraClip prosthesis and acoustic shadowing, which may have contributed to the reduced inter-rater agreement. Nevertheless, regurgitant fractions by Doppler quantification had substantially improved reproducibility over expert readers’ subjective assessment, which underlines the importance of quantitative metrics over-and-above visual qualitative analysis.
CMR phase contrast imaging provided reproducible measurements of trans-mitral blood flow and regurgitant fraction, with excellent inter-observer agreement (13). CMR was possible in all eligible cases, with no patients abandoning due to discomfort or claustrophobia, a limitation which was reported in other studies (32). However, CMR was not feasible in nine out of 25 patients (36%) due to arrhythmia or non-MRI compatible implanted device, reflecting the common co-morbidities of patients undergoing MitraClip interventions.
The use of CMR to measure mitral regurgitant fractions traditionally has used a comparison of SVs in a similar fashion to Doppler echocardiography, using the MRI “stroke volume” technique (RF%MRI-SV) comparing left ventricular SV, derived from cine CMR SSFP imaging, to the aortic forward SV derived from flow CMR imaging (16,27). This methodology has inherent propensity for error, due to comparison of two different techniques for SV analysis (left ventricular SV calculated from cine CMR images, compared to aortic forward SV from flow CMR data). However, this technique had significant underestimation error in some patients with definite mitral regurgitant jets on Doppler imaging, including nonsensical “negative regurgitation” in two cases.
In addition to this standard MRI technique, we applied a novel technique of placing a specific phase-contrast acquisition plane immediately atrial to the MitraClip, thereby measuring forward and reverse through-plane flow in a single breath-held acquisition. This has the potential advantage of reducing observer error and indeed showed excellent reproducibility between readers. However, one disadvantage of this technique was the patient with a “swinging jet” due to leaflet prolapse, which caused the mitral regurgitant flow to no longer be perpendicular to the phase contrast plane and thus unable to be captured in the through-plane phase contrast measurements.
CMR has long been shown to have superior reproducibility over other techniques, thereby substantially reducing numbers needed for clinical trials (33). The present data suggest that, in the same manner, CMR will be useful to test the efficacy of emerging therapies for MR, paralleling its use in clinical trials for heart failure.
Limitations
This small study was performed in a single center with considerable expertise in both echocardiography and CMR. These findings may not be generalized to centers with less expertise in quantitative analysis, or different hardware (e.g., high-field 3.0 T CMR may not be suitable due to increased susceptibility artefacts from the MitraClip). Susceptibility artefact from the MitraClips in the basal left ventricular slice may cause difficulty in defining the endocardium, contributing to errors in the MRI-SV method.
Conclusions
This study shows that quantitation of residual regurgitation after MitraClip percutaneous mitral valve repair can be assessed by CMR, with superior reproducibility compared to Doppler echocardiography. Both techniques, however, have inherent limitations, and quantitation of residual regurgitation after MitraClip remains challenging.
Acknowledgements
Funding: Funding was provided by the Richard Slaughter Centre of Excellence in Cardiovascular Magnetic Resonance, the Smart Futures Fellowship Grant (Department of Science, Information Technology, Innovation and the Arts, Queensland Government #ISF783), and an unrestricted educational grant from Abbott Vascular (with no involvement in the study design, analysis or writing).
Disclosure: The authors declare no conflict of interest.
References
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [PubMed]
- Stein KM, Borer JS, Hochreiter C, et al. Prognostic value and physiological correlates of heart rate variability in chronic severe mitral regurgitation. Circulation 1993;88:127-35. [PubMed]
- Suri RM, Schaff HV, Dearani JA, et al. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovasc Surg 2009;137:1071-6. [PubMed]
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847. [PubMed]
- Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012;59:130-9. [PubMed]
- Armstrong EJ, Rogers JH, Swan CH, et al. Echocardiographic predictors of single versus dual MitraClip device implantation and long-term reduction of mitral regurgitation after percutaneous repair. Catheter Cardiovasc Interv 2013;82:673-9. [PubMed]
- Paranskaya L, D'Ancona G, Bozdag-Turan I, et al. Residual mitral valve regurgitation after percutaneous mitral valve repair with the MitraClip® system is a risk factor for adverse one-year outcome. Catheter Cardiovasc Interv 2013;81:609-17. [PubMed]
- Chan PH, She HL, Alegria-Barrero E, et al. Real-world experience of MitraClip for treatment of severe mitral regurgitation. Circ J 2012;76:2488-93. [PubMed]
- Alegria-Barrero E, Chan PH, Paulo M, et al. Edge-to-edge percutaneous repair of severe mitral regurgitation--state-of-the-art for Mitraclip® implantation. Circ J 2012;76:801-8. [PubMed]
- Foster E, Wasserman HS, Gray W, et al. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol 2007;100:1577-83. [PubMed]
- Myerson SG. Heart valve disease: investigation by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2012;14:7. [PubMed]
- Cawley PJ, Maki JH, Otto CM. Cardiovascular magnetic resonance imaging for valvular heart disease: technique and validation. Circulation 2009;119:468-78. [PubMed]
- Cawley PJ, Hamilton-Craig C, Owens DS, et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ Cardiovasc Imaging 2013;6:48-57. [PubMed]
- Krumm P, Zuern CS, Wurster TH, et al. Cardiac magnetic resonance imaging in patients undergoing percutaneous mitral valve repair with the MitraClip system. Clin Res Cardiol 2014;103:397-404. [PubMed]
- Didier D, Ratib O, Lerch R, et al. Detection and quantification of valvular heart disease with dynamic cardiac MR imaging. Radiographics 2000;20:1279-99; discussion 1299-301. [PubMed]
- American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462-508. [PubMed]
- Devos DG, Kilner PJ. Calculations of cardiovascular shunts and regurgitation using magnetic resonance ventricular volume and aortic and pulmonary flow measurements. Eur Radiol 2010;20:410-21. [PubMed]
- Aurigemma G, Reichek N, Schiebler M, et al. Evaluation of aortic regurgitation by cardiac cine magnetic resonance imaging: planar analysis and comparison to Doppler echocardiography. Cardiology 1991;78:340-7. [PubMed]
- Chatzimavroudis GP, Oshinski JN, Franch RH, et al. Evaluation of the precision of magnetic resonance phase velocity mapping for blood flow measurements. J Cardiovasc Magn Reson 2001;3:11-9. [PubMed]
- Debl K, Djavidani B, Buchner S, et al. Assessment of the anatomic regurgitant orifice in aortic regurgitation: a clinical magnetic resonance imaging study. Heart 2008;94:e8. [PubMed]
- Dulce MC, Mostbeck GH, O'Sullivan M, et al. Severity of aortic regurgitation: interstudy reproducibility of measurements with velocity-encoded cine MR imaging. Radiology 1992;185:235-40. [PubMed]
- Ley S, Eichhorn J, Ley-Zaporozhan J, et al. Evaluation of aortic regurgitation in congenital heart disease: value of MR imaging in comparison to echocardiography. Pediatr Radiol 2007;37:426-36. [PubMed]
- Wittlinger T, Dzemali O, Bakhtiary F, et al. Hemodynamic evaluation of aortic regurgitation by magnetic resonance imaging. Asian Cardiovasc Thorac Ann 2008;16:278-83. [PubMed]
- Myerson SG, d'Arcy J, Mohiaddin R, et al. Aortic regurgitation quantification using cardiovascular magnetic resonance: association with clinical outcome. Circulation 2012;126:1452-60. [PubMed]
- Kahlert P, Plicht B, Jánosi RA, et al. The role of imaging in percutaneous mitral valve repair. Herz 2009;34:458-67. [PubMed]
- Kon MW, Myerson SG, Moat NE, et al. Quantification of regurgitant fraction in mitral regurgitation by cardiovascular magnetic resonance: comparison of techniques. J Heart Valve Dis 2004;13:600-7. [PubMed]
- Chatzimavroudis GP, Oshinski JN, Pettigrew RI, et al. Quantification of mitral regurgitation with MR phase-velocity mapping using a control volume method. J Magn Reson Imaging 1998;8:577-82. [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [PubMed]
- Enriquez-Sarano M, Bailey KR, Seward JB, et al. Quantitative Doppler assessment of valvular regurgitation. Circulation 1993;87:841-8. [PubMed]
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358-67. [PubMed]
- Gelfand EV, Hughes S, Hauser TH, et al. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: optimizing correlation with Doppler echocardiography. J Cardiovasc Magn Reson 2006;8:503-7. [PubMed]
- Orwat S, Diller GP, Kaleschke G, et al. Aortic regurgitation severity after transcatheter aortic valve implantation is underestimated by echocardiography compared with MRI. Heart 2014;100:1933-8. [PubMed]
- Bellenger NG, Davies LC, Francis JM, et al. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2000;2:271-8. [PubMed]





