Mitral valve repair for ischemic mitral regurgitation
Introduction
Overview: mitral valve repair in cardiomyopathy
Since Bolling’s seminal paper more than a decade ago (1) mitral valve repair for mitral regurgitation (MR) in cardiomyopathy remains controversial. The controversy exists primarily in the setting of ischemic cardiomyopathy (2). The pathophysiology of ischemic MR is now accepted to be the result of adverse left ventricular remodeling in the setting of a morphologically normal mitral valve (3). Ventricular dilatation secondary to ischemia leads to lateral papillary muscle displacement which effectively pulls the chordae and the valve leaflets apically (4). This tethering, combined with annular dilation, creates a central leaflet coaptation defect which causes MR in the form of a central regurgitant jet (4). Successful repair of the valve consequently relies on placement of an undersized ring to bring the annulus together and reestablish the coaptation plane (5).
Two distinct questions arise when operative intervention on the mitral valve is considered in the setting of ischemia: (I) should concomitant intervention be undertaken during coronary artery bypass grafting (CABG) and (II) what type of intervention (repair or replacement) should be undertaken.
When MR is moderate, controversy surrounds whether CABG alone to the ischemic myocardium would induce adequate reverse remodeling to restore the subvalvular geometry and consequently diminish or eliminate regurgitation (2). If valve repair accomplishes this, then does valve repair provide any survival advantage? Would this advantage outweigh the increased operative risk associated with concomitant repair?
When MR is severe, the need for valve intervention is not disputed. Rather, controversy surrounds whether repair versus replacement should be undertaken. The answer hinges upon whether elimination of regurgitant volume overload will arrest any further remodeling. If yes, then repair should suffice because the ventricle will not undergo additional remodeling and MR will not recur. If the ventricle continues to remodel despite elimination of regurgitation at the time of operation, then replacement is necessary, as it would only be a matter of time before ongoing LV dilatation and remodeling would induce recurrent MR in the repaired valve.
The Cardiothoracic Surgical Trials Network (CTSN), sponsored by NIH and the Canadian Institutes of Health Research, is attempting to definitively answer these controversies by conducting multicenter, randomized surgical trials within the last seven years. The CTSN recently published short-term results on mitral valve repair versus replacement for severe MR (6). These results demonstrate that at 12 months follow up for severe MR, there was no difference in survival between repair versus replacement, but repair was associated with a much higher recurrence rate (6). The general consensus of the cardiac surgery and heart failure community was that this short-term data supported mitral valve replacement in the setting of severe MR.
For short-term results of CABG versus CABG and mitral valve repair in the setting of moderate MR, the CTNS will be publishing their results very soon.
Operative technique
Operative preparation
Exposure to the mitral valve is achieved via median sternotomy. This is our preferred positioning because of maximum flexibility in addressing any pathology that may be unexpectedly encountered or other concomitant procedures in these patients. Swan Ganz lines are utilized only for patients with poor ventricular function or severe pulmonary hypertension or right heart failure. TEE is utilized with every procedure to assess ventricular function and valvular interventions. We prefer to tuck the patient’s arms while being careful of the ulnar nerve.
Exposition
Operative exposure of the mitral valve: Sondergaard’s groove
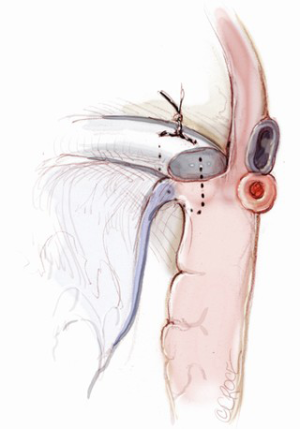
The mitral valve is the hardest of the four heart valves to expose. Why is this? Anatomically, the mitral valve is furthest from the operating surgeon when approached from the right side of the operating table. The valve is deep and inferior to the surgeon; furthermore, in its natural anatomic state, it cannot be viewed en face (Figure 1). Proper exposure of the mitral valve consequently hinges upon understanding and manipulating this anatomy. Exposing the valve so that the surgeon sees it en face requires more distortion of the natural anatomy of the heart than any other valve. To achieve this en face view, the heart should be rotated counterclockwise (looking down from the ceiling), and the apex should be pushed posteriorly and into the left pleural space as much as possible. This positioning is augmented by bringing up the right pericardium with stay sutures and facilitated by releasing any left pericardial stay sutures. In addition to achieving this critical en face view of the valve, these maneuvers will rotate the valve orifice such that the surgeon is looking directly into the LV cavity through an en face view of the mitral valve.
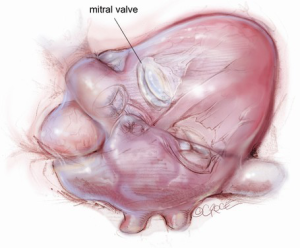
The first step in this exposure is as complete a development of Sondergaard’s groove, between the right and left atria (Figure 2) as much as possible. Conceptually, from the operating surgeon’s perspective, the right and left atria should be viewed as the anterior and posterior atria with respect to the groove. The surgeon is lifting the “anterior” right atrium off the “posterior” left atrium through a combination of blunt and electrocautery dissection. With the Bovie electrocautery set to low, development of the groove should be performed approximately 2-3 cm from the right superior pulmonary vein up to the atrial septum near the fossa (Figure 3). At this location, there is no plane to dissect and the dissection should stop. Next, an incision should be made into the top of the left atrium and care should be taken to maintain a distance of at least 1 cm from the right superior pulmonary vein in order to minimize the risk of injury to the vein (Figure 4). The incision should extend inferiorly between the IVC and the right inferior pulmonary vein and superiorly toward the center of the left atrial dome. Particular care should also be taken to avoid extending the atriotomy too high up and underneath the aorta, making a secure closure hazardous. In elderly female patients, this must be done very carefully. A left atrial dome tear, which continually propagates toward the left atrial appendage underneath the aorta ultimately requires repeat cross clamping and transection of the aorta and SVC for adequate repair, adding considerable morbidity and mortality to the procedure.
Once an atriotomy is made, further maneuvers are performed to facilitate an en face view of the mitral valve and to minimize the distance between valve and surgeon, include raising the head and tilting the left side of the operating table down. The superior vena cava inherently tethers the heart down and hinders the dome from being lifted superiorly to expose the valve through Sondergaard’s groove. To counter this tethering, the SVC pericardial attachments may be divided and dissected superiorly (Figure 5), while understanding the phrenic nerve is on the other side of the pericardium. To facilitate moving the apex towards the left, the left pericardium can be opened and the ventricular apex pushed into the left pleural space with a sponge, thereby rotating the mitral annular plane toward the surgeon’s position (Figure 6). Of course, the best exposure may be obtained by complete transection of the SVC, but this is very rarely required. Finally, an additional maneuver is to place sponges on the LV anterior wall to physically push the apex of the heart down and away, moving the mitral valve up and toward the operating surgeon.
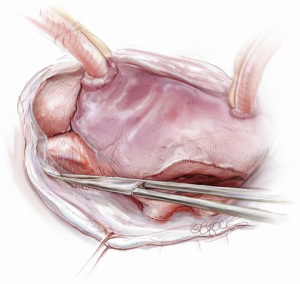
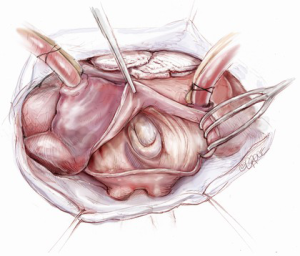
Operative exposure of the mitral valve: other approaches
Alternative exposures of the valve include a transseptal incision or approach via right thoracotomy. The transseptal incision has the advantage of allowing the surgeon to be closer to and right on top of the mitral valve. The disadvantage is the extra incision as well as greater postoperative conduction issues created by the extra incision in the right atrium (7). For patients with an extended antero-posterior (AP) dimension, the transseptal approach may be necessary because a large AP dimension accentuates the anatomic features that make mitral valve exposure difficult to begin with. For the right thoracotomy exposure, the advantage is avoiding the front (especially useful in the reoperative setting) and the fact that the surgeon, peering through the right rib cage, is more en face naturally than from the standard sternotomy. The disadvantage is complexity of bypass access and the much greater distance to the valve for the surgeon.
Operative exposure in cardiomyopathy
In dilated cardiomyopathy, the heart is large by definition and the left atrium is likely to be quite large as well. Because of this, there is rarely difficulty in exposing the mitral valve, given the remodeled myocardial substrate. Difficulty in mitral valve exposure occurs in patients with large AP dimension and a small left atrium, none of which typically occurs in cardiomyopathy. The authors have personally never been required to utilize the transseptal approach for cardiomyopathy patients.
Cardiopulmonary bypass
We typically utilize bicaval cannulation through the atria for better drainage compared to single stage cannulation. The arterial cannula is placed into the distal ascending aorta. We recommend both antegrade and retrograde cardioplegia given the need for meticulous protection in the setting of cardiomyopathy as well as the fact that concomitant coronary disease is present by definition when addressing ischemic functional MR. Once bypass has commenced, both cavae are placed to the patients left side. This helps to expose the approach to Sondergaard’s groove.
Operation
Assessment of valve dysfunction and repair strategy
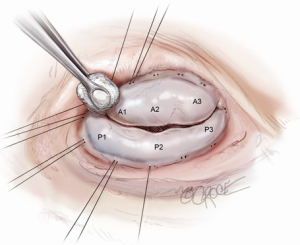
Proper evaluation of the valve is critical to a successful repair (4) and begins prior to cardiac arrest with transesophageal echocardiography (TEE). The height of the anterior leaflet from the hinge point to the coaptation plane is measured. Once the valve is exposed, the leaflets should be examined closely to verify that they are morphologically normal and TEE findings should be confirmed by insufflating the ventricle with saline to test the valve and directly observe the central regurgitant jet. As noted previously, in contrast to degenerative or myxomatous disease that directly affects leaflet integrity and morphology, ischemic FMR results from a distortion and dilation of native ventricular geometry that normally supports normal leaflet coaptation. To counter this, the first and most crucial step in successful valve repair is placement of an undersized, complete remodeling annuloplasty ring to restore the annulus to its native geometry.
Annulus sizing and annuloplasty
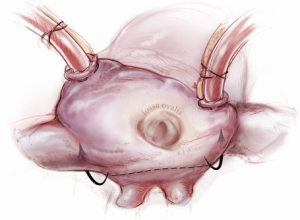
Sizing is performed to the anterior annulus using a right angle placed in the right hand to pull the anterior chordae down and therefore pull the entire anterior leaflet down. The sizer is introduced with the left hand. Cutting out a notch in the sizers allows simultaneous placement of sizer and right angle in the operative field (Figure 7). Sizing is done with the goal of reestablishing the coaptation plane between anterior and posterior leaflet. One undersizes to bring the annulus together and the leaflets into alignment (Figure 8). While both partial (C-ring) and complete (O-ring) annuloplasty rings are currently described in the literature, we recommend that only a complete ring be used to treat ischemic functional MR to avoid recurrence as the anterior annulus may dilate as well (8). The anterior annulus does not dilate as much or frequently as the posterior annulus, but indeed it does dilate (8). Furthermore, the three-to-four additional anterior annular sutures required with a complete ring adds very little to procedure time and complexity.
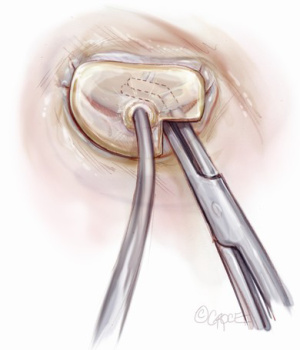
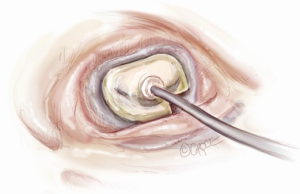
A multitude of rings have been designed for the various annular geometries encountered in ischemic functional MR. In our experience, however, no consistent annular geometry predominates and with so much variation in individual anatomy and ventricular dilation, these rings add very little to the concept of repair. Rather, we continue to emphasize that a complete, undersized ring is of paramount importance to successful repair and will outweigh any minute advantage gained by employing a specially designed ring.
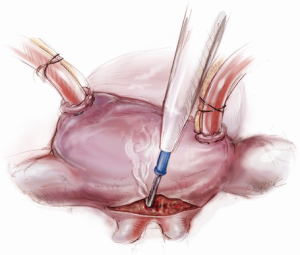
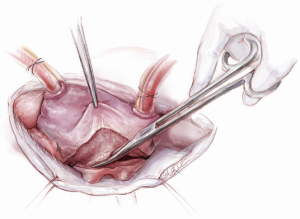
Initial annuloplasty sutures should begin at P2 and move clockwise to the middle of the anterior leaflet. Following these sutures, the next set should begin again at P2 this time travelling counterclockwise. Travelling around the annulus, the P1 stiches and the anterolateral trigone stitches are the most difficult to place. Placement of these sutures is facilitated by using the uncut ends of previous sutures to pull down the annulus while using a “peanut” to retract the atrium up and off the trigone (Figure 9) in order to expose it. Additionally, sutures leading up to the trigone should be placed forehand with the surgeon’s palm facing the opposite wall. In this orientation, the needle should first be directed into the left ventricle and then turned back toward the annulus. Of critical importance during annuloplasty is avoiding the circumflex artery. This can be avoided by meticulously passing sutures in a plane parallel to the plane of the artery (Figure 10). In this way, when the stitches are tied, no radial component of force to the circumflex artery will be present to distort the artery. After all sutures are placed, the valve should be passively tested prior to closure of the atriotomy.
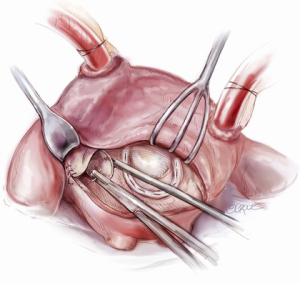
The aortotomy closure superiorly may be difficult, especially if the left atrial incision has torn superiorly during retraction. This is a common occurrence. At times, placing the first stitch out to in is impossible because of the overlying SVC and aorta. In this situation, the first stitch should be placed in to out, with the first knot being tied inside the left atrium. A dental mirror may be necessary to see the suture line from the inside and to ensure its integrity (Figure 11). Regardless of how it is performed, the superior incision must be closed meticulously and securely, because any tearing of the LA suture line underneath the aorta after removal of the cross clamp will typically necessitate re-arresting the heart to adequately address the problem. In an elderly female, this can have disastrous and fatal implications. Rarely, felt may be used to help buttress the fragile left atrial tissue in closing.
Operation conclusion and postoperative care
At the conclusion of the procedure, all suture lines should be meticulously checked, especially the LA suture line. Repair stitches to the suture line should be performed preferably with pledgeted mattress stitches. When performing mattress stitch repairs to the LA suture line, our preference is to use an 3-0 or 4-0 Prolene on an SH needle. Consideration should be given to inotropic support when weaning from bypass, given that repair of the valve may unmask further ventricular dysfunction. In the ICU, postoperative care is routine.
Conclusion: mitral valve repair in cardiomyopathy
Once regarded as anathema, mitral valve repair in cardiomyopathy is now clearly accepted as a viable treatment option for MR. The recent CTSN trial for severe MR suggests that valve replacement is the treatment of choice for severe MR, but that for moderate MR, valve repair by annuloplasty will continue to have an important role. When repair is appropriate, excellent results can be achieved with undersized, complete ring annuloplasty.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bolling SF, Deeb GM, Brunsting LA, et al. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg 1995;109:676-82;discussion 682-3. [PubMed]
- Kwon MH, Cevasco M, Chen FY. Functional, ischemic mitral regurgitation: to repair or not to repair? Circulation 2012;125:2563-5. [PubMed]
- Schmitto JD, Lee LS, Mokashi SA, et al. Functional mitral regurgitation. Cardiol Rev 2010;18:285-91. [PubMed]
- Connell JM, Worthington A, Chen FY, et al. Ischemic mitral regurgitation: mechanisms, intraoperative echocardiographic evaluation, and surgical considerations. Anesthesiol Clin 2013;31:281-98. [PubMed]
- Kwon MH, Lee LS, Cevasco M, et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg 2013;146:616-22. [PubMed]
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32. [PubMed]
- Lukac P, Hjortdal VE, Pedersen AK, et al. Superior transseptal approach to mitral valve is associated with a higher need for pacemaker implantation than the left atrial approach. Ann Thorac Surg 2007;83:77-82. [PubMed]
- Kwon MH, Lee LS, Cevasco M, et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg 2013;146:616-22. [PubMed]





