Minimally invasive mitral valve repair through right minithoracotomy in the setting of degenerative mitral regurgitation: early outcomes and long-term follow-up
Introduction
Degenerative mitral valve (MV) regurgitation is the most common valve disease in the developed countries, with an estimated annual incidence of 2-2.5% (1,2). Left untreated or solely under medical treatment, severe MV regurgitation leads to heart failure, with a mortality rate of 6-7% each year (3).
In this setting, MV repair is the operation of choice for degenerative mitral regurgitation. Compared with replacement, MV repair is associated with better postoperative outcomes, long-term survival and long-term durability, restores the normal life expectancy and quality of life, and reduces the rate of endocarditis and thromboembolic events (4). The most common procedure used to perform MV repair is full sternotomy; however, in the last decade, several minimally invasive mitral valve surgery (MIMVS) approaches have been developed to reduce the invasiveness of surgical procedures, offering comparable safety and quality to the conventional approach (5-9). Compared with conventional procedure, several meta-analysis have shown that MIMVS is associated with excellent postoperative outcomes, particularly mortality and morbidity (10-13). Despite these results, critics have claimed that it is technically more demanding, has a longer learning curve, requires dedicated instruments, and reduces the chances of MV repair in favor of replacement for complex degenerative valve disease (14). Since 2003, MIMVS has become our first approach to treat MV surgery. The aim of our study is to evaluate the early and long-term outcomes of patients undergoing MV repair through right minithoracotomy for severe degenerative MV regurgitation.
Methods
Patients and data collection
A retrospective observational study was undertaken from prospectively collected data on 1,171 consecutive patients undergoing MIMVS through right thoracotomy between September 2003 and December 2011. The ethics committee approved the study, and individual consent was waived. The data collection form was entered in a local database and included three sections completed consecutively by the cardiac surgeons, anesthetists and perfusionists involved in patient’s care. Patients were excluded if they had non-degenerative MV regurgitation (n=364) or underwent a MV replacement directly despite the degenerative etiology (n=94). Criteria for MVR for MV regurgitation were the presence of heavy calcification on annulus and/or leaflets, and age >80 years with complex repair or left ventricular dysfunction. The final sample size included 703 patients, of whom 670 were successfully repaired (95.3%). Patients undergoing MV replacement after failure of MV repair were considered as intention to treat for early outcome analysis. These patients were excluded from long-term analysis follow-up. Early mortality was defined as any death occurring within 30 days of operation or before discharge from the hospital. Stroke was defined as any new focal or global neurological deficit lasting more than 24 hours detected by computed tomography or nuclear magnetic resonance imaging, and confirmed by neurologists or neuroradiologists. Acute kidney injury was defined as postoperative creatinine increase of 1+ mg/dL or requirement of hemodialysis. Perioperative myocardial infarction was defined as new Q waves >0.04 ms and/or reduction in R waves >25% in at least two contiguous leads on ECG.
All patients were seen eight to 12 weeks postoperatively and thereafter were contacted for follow-up data. The median follow-up time was 48 months [interquartile range (IQR) 31-76 months] and the follow-up data were 96% complete.
All patients had transthoracic echocardiography done preoperatively and on discharge. The severity of mitral regurgitation was graded based on the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery recommendations (15). Echocardiographic follow-up consisted of those patients who survived and had postoperative echocardiogram at least six months (329 patients, 49.1%,) at a median of 42 months (IQR 10-71), after surgery. Recurrent MR was defined as moderate or severe on a four-point grade: trivial, 1/4; mild, 2/4; moderate, 3/4; and severe, 4/4.
Surgical technique
The surgical approach for MIMVS has been reported elsewhere (13). Briefly, MIMVS through a right anterior thoracotomy is performed through a 5-7 cm skin incision placed at the third or fourth intercostal space. After incision, two ports are inserted in the thorax to allow positioning of a ventricular vent, CO2 insufflator, camera device and pericardial stay sutures. A soft tissue is inserted and ribs are spread gently with a small retractor. After heparin administration, a percutaneous venous cannula is inserted through the femoral vein into the superior vena cava, under transesophageal echocardiographic guidance and using the Seldinger technique. Direct aortic cannulation is performed using flexible cannulae, whilst the arterial pressure is reduced. After vacuum-assisted cardiopulmonary bypass (CPB) is established (–40 to –60 mmHg), the patient is cooled to 34 °C and the ascending aorta is clamped with the aortic Detachable Glauber clamp (Cardiomedical GmbH, Langenhagen, Germany; distributed by Sorin, Salluggia, Italy). Antegrade cold crystalloid or warm blood cardioplegia is delivered directly into the ascending aorta by a needle vent catheter.
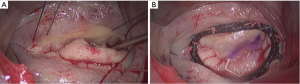
The MV is approached through the Sodengaard grove and exposed using a specially designed atrial retractor held by a mechanical arm inserted through a right parasternal port. MV procedures are performed under a combination of direct vision and thoracoscopic assistance. Figure 1 shows a MV prolapse on P2 treated with P2 triangular resection, neochordae on P2 and A2 and insertion of a semi-rigid closed prosthetic ring. Eight surgeons contributed to this series, with two of them (MG, MS) performing 64% of the operations.
Statistical analysis
Continuous data were expressed as mean ± standard deviation or median with the IQR and categorical data as percentages. Cumulative survival was evaluated with the Kaplan–Meier method. All reported P values are two-sided, and P values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed with SPSS 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics are listed in Table 1. The mean age was 62±13 years, 295 (42%) patients were female and 16 (2.3%) had previous cardiac operations.
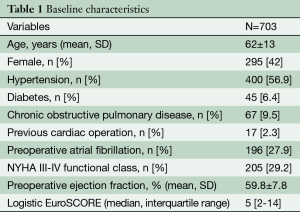
Full table
MV repair was successfully performed in 670 patients, with a rate of success of 95.3%. Repair techniques included annuloplasty (n=632), either alone (n=196) or associated with other repair techniques (n=436), including leaflet resection (n=381), neochordae implantation (n=84), and sliding plasty (n=74).
The mean CPB and cross-clamp times were 137±43 minutes and 94±37 minutes respectively. Concomitant procedures included tricuspid valve repair (n=77, 11%), atrial fibrillation ablation (n=70, 10%) and atrial septal repair (n=24, 3.4%). In one case, mitral repair was combined with an aortic valve replacement.
Direct aortic cannulation was achieved in 610 (86.8%) patients, whereas the endoaortic ballon was used in 62 patients (8.8%). Finally, 6 (1%) operations were performed in beating heart/ventricular fibrillation, as shown in Table 2.
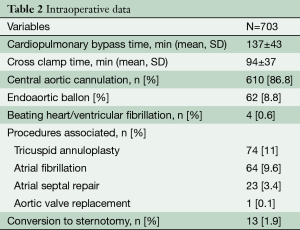
Full table
Perioperative outcomes
Overall in-hospital mortality was 0.1% with a predicted median EuroSCORE of 5% (IQR 2-14.3%) (n=1: this patient died for multi-organ failure due to sepsis). Thirteen patients (1.8%) had conversion to full sternotomy because of bleeding (n=10, 1.4%) or for strong pleural adhesions (n=3, 0.5%). Reoperation for bleeding was 4.4% (n=31) whereas incidence of stroke and acute renal failure requiring dialysis were 1.3% (n=9) and 1% (n=6), respectively. Median ventilation time, ICU stay and ward stay were seven hours (IQR 5-10 hours), one day (IQR 1-1 day), and six days (IQR 5-8). Sixty-nine percent (n=488) of patients was discharged home, as shown in Table 3. After repair, residual MR at discharge was very low: no MR: (n=506, 71.8%), trivial: (n=140, 19.9%), mild: (n=24, 3.4%), moderate or severe MR: (n=1, 0.1%).
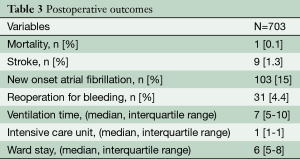
Full table
Survival, freedom from reoperation and residual MR at follow-up
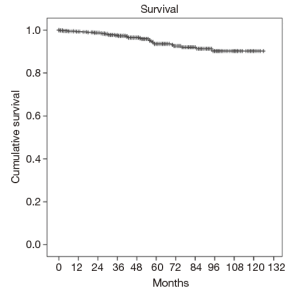
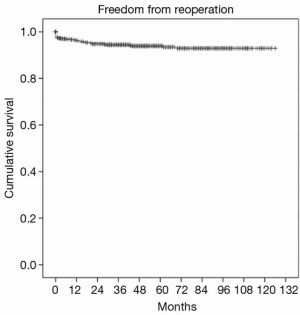
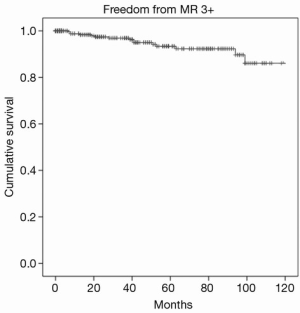
Overall one, five and eight-year survival after MV surgery was 99.3%, 93.6%, and 90.1% respectively, as shown in Figure 2. Moreover, one-, five-, and eight-year freedom from reoperation was 96%, 94%, and 93%, respectively, as shown in Figure 3. Finally, freedom from recurrent mitral regurgitation was 99% at one year, 93% at five years and 90% at eight years, shown in Figure 4.
Discussion
Our study demonstrates that MV repair through MIMVS is a safe and feasible procedure associated with excellent early and long-term follow-up. Specifically, overall mortality and stroke rate was 0.2% and 1.3%, respectively and survival at eight years was 90%. Moreover, the rate of MV repair at discharge was 95.3%, higher than data reported by the Society of Thoracic Surgeons (STS) database (6). Finally, freedom from reoperation and mitral regurgitation were 92% and 90% at eight-year follow-up. These data are in line with previous studies, confirming that MIMVS may offer the same quality and safety of a conventional approach, guaranteeing high rate of MV repair, even in complex cases (16-20). Despite a number of studies showing at least equivalent surgical results compared with the conventional approach, traditionalists claim that MV repair through minithoracotomy is more complex and technically challenging, especially in the presence of Barlow’s MV disease (11,14,16,19). As a result, MIMVS may reduce the rate of MV repair. However, a meta-analysis of 2,000 patients has shown satisfactory echocardiographic outcomes, and the incidence of moderate/severe mitral regurgitation was 0.1% in the MIMVS group and 0.3% in the conventional sternotomy group, respectively (12). Our data are in line with this study, reporting a rate of moderate mitral regurgitation of 0.1% at discharge. Furthermore, our long-term freedom from reoperation was 93%, confirming that this approach does not influence the outcome of MV repair. A second criticism concerns the learning curve required for both MV repair and minithoracotomy approach; however, a young surgeon should intuitively start approaching the MV repair through standard sternotomy, and progress toward less invasive techniques thereafter. In this setting, the learning curve requires two important steps, raising ethical concerns regarding the safety of patients and their outcomes. Recently, we investigated the operative outcomes of five young surgeons who were trained in MV repair directly through a minimally invasive approach, and a senior surgeon (MG) who introduced the technique and was responsible for the training program. Interestingly, we found no statistical difference in terms of mortality, morbidity and rate of MV repair, concluding that MIMVS repair is safe and reproducible technique that can be taught successfully to cardiac trainees (21). Similar results were achieved by the Leipzig group (22). Finally, according to a cross-sectional survey on MIMVS, Misfeld and colleagues concluded that more than 20 cases are required to gain familiarity with less invasive techniques (23). The third criticism concerns the increased risk of stroke associated with MIMVS; however, the stroke rate in our study (1.3%) was similar to those reported in the literature for conventional standard sternotomy (6). We recently demonstrated that antegrade perfusion with direct aortic cannulation reduces the risk of neurological events compared with peripheral cannulation, avoiding morbidities related to groin cannulation such as wound dehiscence and pseudoaneurysms (24).
Finally, for many surgeons, the decision to utilize MIMVS is more related to the cosmetic results than better clinical outcomes, because no large randomized trial has been performed. However, the chance to perform a well-designed randomized trial with appropriate sample size is difficult as MIMVS has guaranteed the same quality and safety of the standard approach, and patients now demand less invasive procedures, especially in well-known minimally invasive centers.
This study has several limitations. It is based on a retrospective analysis of patients undergoing consecutively MIMVS over the eight-year period and potential bias might be present. However, our database was filled in prospectively. Secondly, our database was not able to distinguish between Barlow MV disease and fibroelastic disease, and no information was reported on anterior or posterior leaflet disease. Thirdly, our echocardiographic follow-up was only completed by 70% of the patient population, which may have influenced results. Finally, because MIMVS is our first approach to treating MV disease, we were not able to perform a retrospective study comparing patients undergoing conventional surgery versus the minithoracotomy approach.
In conclusion, in the setting of degenerative MV regurgitation, our study demonstrates that MIMV repair through right minithoracotomy is a safe and reproducible procedure associated with high rate of MV repair, and excellent early postoperative and long-term results.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr Glauber, Dr Ferrarini and Dr Solinas have disclosures with Sorin Group.
References
- Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet 2009;373:1382-94. [PubMed]
- Freed LA, Levy D, Levine RA, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1-7. [PubMed]
- Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med 2009;361:2261-9. [PubMed]
- Wong RH, Lee AP, Ng CS, et al. Mitral valve repair: past, present, and future. Asian Cardiovasc Thorac Ann 2010;18:586-95. [PubMed]
- Schmitto JD, Mokashi SA, Cohn LH. Minimally-invasive valve surgery. J Am Coll Cardiol 2010;56:455-62. [PubMed]
- Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8,1410.e1; discussion 1408-10.
- McClure RS, Athanasopoulos LV, McGurk S, et al. One thousand minimally invasive mitral valve operations: early outcomes, late outcomes, and echocardiographic follow-up. J Thorac Cardiovasc Surg 2013;145:1199-206. [PubMed]
- Modi P, Rodriguez E, Hargrove WC 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7. [PubMed]
- Davierwala PM, Seeburger J, Pfannmueller B, et al. Minimally invasive mitral valve surgery: "The Leipzig experience Ann Cardiothorac Surg 2013;2:744-50. [PubMed]
- Ritwick B, Chaudhuri K, Crouch G, et al. Minimally invasive mitral valve procedures: the current state. Minimally Invasive Surgery 2013;2013:8.
- Cao C, Gupta S, Chandrakumar D, et al. A meta-analysis of minimally invasive versus conventional mitral valve repair for patients with degenerative mitral disease. Ann Cardiothorac Surg 2013;2:693-703. [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [PubMed]
- Ding C, Jiang DM, Tao KY, et al. Anterolateral minithoracotomy versus median sternotomy for mitral valve disease: a meta-analysis. J Zhejiang Univ Sci B 2014;15:522-32. [PubMed]
- Cooley DA. Minimally invasive valve surgery versus the conventional approach. Ann Thorac Surg 1998;66:1101-5. [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. [PubMed]
- Borger MA, Kaeding AF, Seeburger J, et al. Minimally invasive mitral valve repair in Barlow's disease: early and long-term results. J Thorac Cardiovasc Surg 2014;148:1379-85. [PubMed]
- Perier P, Hohenberger W, Lakew F, et al. Rate of repair in minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:751-7. [PubMed]
- Reser D, van Hemelrijck M, Pavicevic J, et al. Repair rate and durability of video assisted minimally invasive mitral valve surgery. J Card Surg 2014;29:766-71. [PubMed]
- Muneretto C, Bisleri G, Bagozzi L, et al. Results of minimally invasive, video-assisted mitral valve repair in advanced Barlow's disease with bileaflet prolapse. Eur J Cardiothorac Surg 2015;47:46-50; discussion 50-1. [PubMed]
- Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748-56. [PubMed]
- Murzi M, Miceli A, Cerillo AG, et al. Training surgeons in minimally invasive mitral valve repair: a single institution experience. Ann Thorac Surg 2014;98:884-9. [PubMed]
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [PubMed]
- Misfeld M, Borger M, Byrne JG, et al. Cross-sectional survey on minimally invasive mitral valve surgery. Ann Cardiothorac Surg 2013;2:733-8. [PubMed]
- Murzi M, Cerillo AG, Miceli A, et al. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: a propensity score analysis on 1280 patients. Eur J Cardiothorac Surg 2013;43:e167-72. [PubMed]