Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review
Introduction
While thymoma is a rare disease, it remains the most common primary mediastinal neoplasm in adults, with an estimated incidence of 0.15 cases per 100,000 (1). The overwhelming majority of thymic neoplasms are benign and slow-growing, and metastases are typically limited to the pleura, pericardium and/or diaphragm (1). Therefore, complete surgical resection is accepted as the mainstay of therapy, with median sternotomy currently considered to be the gold standard for resection approaches (2,3). However, interest has grown in minimally invasive surgical approaches, most notably video-assisted thoracoscopic surgery (VATS) thymectomy, as a means of reducing perioperative morbidity and mortality (3).
Recent institutional studies have associated VATS thymectomy with improved outcomes, including reduced postoperative pain, fewer complications such as bleeding and pneumonia, shorter hospital stays, better preservation of baseline pulmonary function, and superior cosmesis with the use of smaller surgical incisions (2,4). Furthermore, similar survival and recurrence rates have been demonstrated for VATS thymectomy compared to open thymectomy patients, reinforcing the rising popularity of the VATS approach as it continues to become increasingly used in centers worldwide (2,4).
Nonetheless, evidence for the efficacy, particularly long-term oncological outcomes, of VATS thymectomy compared to open surgery remains limited, with a current paucity of randomized controlled trials. The present systematic review thus aimed to summarize existing studies comparing VATS thymectomy to open thymectomy (transsternal or transthoracic). The primary outcomes of interest were overall and recurrence-free survival, while secondary endpoints included the incidence of postoperative complications and length of hospital stay.
Methods
Literature search
The present systematic review was performed according to recommended PRISMA guidelines (5,6). Six electronic databases, including MEDLINE, EMBASE, PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), and Database of Abstracts of Reviews of Effects (DARE), were searched from their dates of inception to April 2015. To maximize the sensitivity of the search strategy, the following terms were combined: (VATS OR thoracoscopic OR thoracoscopy) AND (open OR sternotomy OR transsternal OR transthoracic) AND (thymus or thymoma or thymic or thymectomy) as either keywords or MeSH terms. The reference lists of articles retrieved were also reviewed in order to identify additional related studies.
Eligibility criteria
Comparative studies that reported any postoperative outcome of VATS thymectomy versus open (transsternal or transthoracic) thymectomy for thymoma were eligible for analysis. At least ten adult patients aged 18 years and over were required to be in each arm of the study. When institutions published duplicate studies with overlapping sample populations, only the most recent reports were included. Only studies published in the English language were selected. Case reports, conference abstracts, editorials, commentaries, pediatric or adolescent studies, and review articles were excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two independent investigators (A.X., R.T.) reviewed each article retrieved. Inter-reviewer discrepancies were resolved by discussion and consensus (A.X., R.T., K.P.). If the study reported medians and ranges we calculated the equivalent means and standard deviations (SDs) using the conversion method described by Hozo, Djulbegovic & Hozo [2005] (7). The studies were also qualitatively assessed using the critical review checklist formulated by the Dutch Cochrane Group and MOOSE guidelines (8) (Table S1). These checklist criteria included the clear definition of study population, outcomes and outcomes assessment; independent outcomes evaluation; adequate follow-up duration; no selective losses to follow-up; and identification of key confounders. The final results were reviewed by the senior investigator (T.D.Y.).
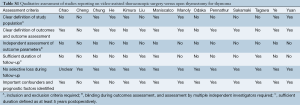
Full table
Statistical analysis
Conventional descriptive statistics were used to summarize the baseline demographics of included patients. Data were presented as raw numbers, percentages, or means with standard deviations unless otherwise indicated. Pooled averages were calculated for outcomes reported in at least three of the included studies. When not explicitly reported in the article text, rates of overall survival and recurrence-free survival were reconstructed for specific time points on digitized Kaplan-Meier curves using the software program, DigitizeIt v2.0.
Results
Quantity and quality of evidence
A total of 414 records were identified through the database searches. After eliminating duplicates and screening the studies based on abstracts 33 full-text articles were assessed using the eligibility criteria. Fourteen relevant studies were selected for analysis, all of which were observational, though two of these trials also used propensity score-matched groups (9,10) (Figure S1, Table 1).
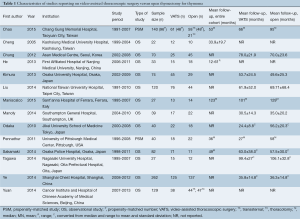
Full table
A total of 1,061 patients were included in the analysis, with 540 undergoing VATS and 521 for open thymectomy. Individual sample sizes varied across the studies, with a median of 23.5 [12-125] for VATS and 22 [10-137] for open thymectomy. The mean length of follow-up similarly varied but was generally longer for open surgery, with a range of 24.4±8.8 to 99.4±27 months for VATS, and 35.0±20.2 to 106.1±32.8 months for the open approach. One study used a historical open thymectomy group (from 2000 to 2005) for comparison with VATS outcomes (2005-2008) (11).
Of the seven studies (9,11-17) that reported their eligibility criteria for either surgical approach, the majority limited their use of the VATS approach to those patients with a tumor diameter of less than 5 cm (12,14,16,17) or 6 cm (13), tumors located inferior and separate to the innominate vein (12,13,16), and/or little or no evidence of invasion or close proximity to vital organs including the heart and great vessels (9,11-16). Exceptions to these criteria, particularly tumors measuring over 5 cm that were still accessible through thoracoscopy, were reported by at least two studies (12,14). The first included study that used propensity-scores matched the VATS and open surgery groups for thymoma Masaoka stage and tumor size (9). The second such study matched for variables of tumor size, presence of myasthenia gravis (MG), and date of surgery (10).
Baseline characteristics
The baseline demographics are summarized in Table 2. The weighted mean age, proportion of male patients, and body mass index (BMI) were similar between VATS and open groups. The percentage of tumors classified as Masaoka stage I (60.2% vs. 58.6%) and II (39.6% vs. 40.1%) was also similar between VATS and open thymectomy patients, respectively. Only one study included thymomas in Stage III (VATS, n=0; open, n=5) and IV (VATS, n=1; open, n=1) (12). Histological grading (A-B3) according to World Health Organization guidelines was additionally comparable between the two groups, with only one study including one open thymectomy patient classified as grade C (17). Mean tumor size, as determined by either computed tomography or histopathology, was slightly larger at 5.6 cm (3.4-7.7 cm) in the open surgery group compared to 4.5 cm in the VATS group (3.2-6.6 cm).
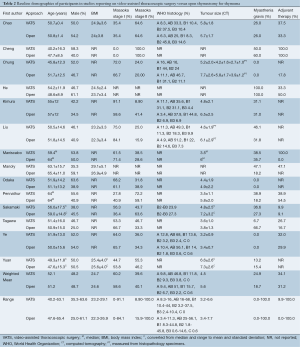
Full table
Furthermore, a higher proportion of VATS patients had MG (24.9%) compared to open thymectomy patients (18.7%), although the range for both groups was large (0-100%), due to several studies incorporating MG into their inclusion (18) or exclusion criteria (11,12,17). The perioperative use of adjuvant therapy (radiotherapy and/or chemotherapy) appeared to be comparable in both groups (34.1% for VATS vs. 31.2% for open), although the range was similarly large (0-100%) across the studies.
Intraoperative characteristics
Differences were noted in the operative approaches and strategies for both VATS and open thymectomy across included studies (Table 3). Based on weighted means the majority of patients underwent a total or extended thymectomy with a higher proportion in the open thymectomy group (98.5%, range, 63.6-100%) than in the VATS group (79.9%, 40.8-100%). Correspondingly, a greater percentage of VATS patients underwent a ‘partial’ thymectomy (20.8%, 0-59.2%) compared to open thymectomy patients (1.5%, 0-36.4%). In the two studies that performed hemithymectomy or partial thymectomies for selected patients, the extent of resections was not clearly defined (9,15).
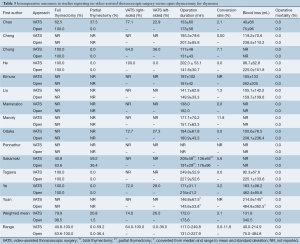
Full table
Most of the unilateral VATS thymectomy patients underwent a right-sided procedure (74%, 64-100%) rather than left-sided (26%, 0-36%). Four studies specified their surgical approach as always being from the side of the tumor (9,11,17,19), while an additional two studies preferred the right-sided approach except in cases of obvious left-sided thymoma (18,20). Maniscalco et al. [2015] reported that their VATS approach was mostly left-sided, although specific rates of use were not included (21). Eight studies included bilateral VATS approaches (9-11,13-16,19), while Tagawa utilized a cervico-xyphoidal-thoracic approach in an unspecified proportion of their patients (16). All open surgery groups utilized a midline sternotomy (n=474) except for a small proportion from the study by Yuan et al. [2014], which utilized a thoracotomy (n=47) (19).
The mean operative duration was similar between VATS (172 minutes, 117.0-249.8) and open thymectomy (173.6 minutes, 131.0-227.9) patients. The conversion rate for VATS was relatively low (3.1%), though this ranged from 0% to up to 11.8% in one study (22). Mean intraoperative blood loss was observed to be markedly higher in the open surgery group (340.5 mL, 75.0-484.8) compared to the VATS group (131.8 mL, 40.0-214.9). There were no cases of intraoperative mortality reported.
Postoperative characteristics
The postoperative outcomes are summarized in Tables 4-6. The length of hospital stay was, on average, longer for open surgery patients (9.8 days, 5.4-19.0) compared to VATS patients (7.0 days, 2.6-14.0). The length of intensive care unit (ICU) stay was similar between the two groups (1.4 days for VATS vs. 1.5 days for open), though this value should be interpreted with caution due to the large variation reported in individual studies. Both the duration of chest drainage and volume drained were lower in VATS patients compared to open thymectomy patients (3.6 vs. 4.8 days, and 408.4 vs. 732.1 mL respectively). Thirty-day mortality was low, at 0% for VATS, and 0.3% for open surgery.
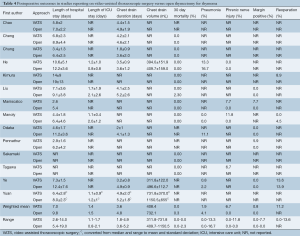
Full table
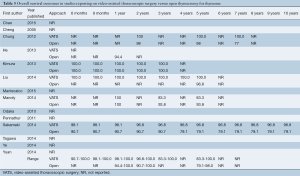
Full table
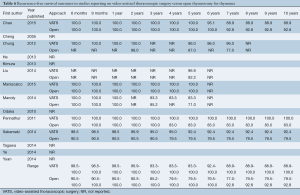
Full table
Complications were variably reported, but higher rates of pneumonia were observed in the open group (4.1% vs. 1.9%), while the incidence of phrenic nerve injury was higher in VATS (6.7% vs. 0% in open surgery). A small proportion (0.8%) of VATS patients also demonstrated positive margins in resected specimens in contrast to none in the open surgery group. Other complications were insufficiently reported and thus not included in the analysis. The rate of reoperation for the VATS group was variable (0-13.6%) with an average of 11.2%, whilst it was inadequately documented for open surgery and therefore not reported.
The ranges of overall survival at 1, 2, and 5 years were similar or higher in VATS patients compared to open thymectomy patients (98.1-100%, 96.8-100%, and 83.3-100% in VATS vs. 94.4-100%, 90.7-100%, and 79.1-98.0% in open surgery, respectively) (Table 5). Small variations in survival rates are most likely attributable to inconsistent reporting and different time points documented across studies, with only four trials that examined 5-year survival (12,14,15,22). Recurrence-free survival at 6 months, 9 months, and yearly for up to 10 years was also predominantly similar or higher for VATS compared to the open surgery group (Table 6). In addition, this outcome was better reported in the studies analyzed, with seven trials that included recurrence-free survival up to at least 5 years (9,10,12,14,15,21,22). Only three studies reported recurrence-free survival at 10 years postoperatively (88.9-100% in VATS vs. 79.5-92.8% in open thymectomy) (9,10,15).
Discussion
Our results suggest that VATS thymectomy may be associated with similar, if not superior, overall and recurrence-free survival rates compared to open surgery. The VATS approach was also shown to potentially result in fewer complications such as bleeding and pneumonia, and shorter hospital stays. Furthermore, the average conversion rate was relatively low, at 3.1%. Subsequently, these findings reinforce those of existing reviews of the literature, though no previous publications have specifically focused on comparing VATS with open thymectomy for thymoma (1,2).
The majority of baseline characteristics, including demographics, use of adjuvant radiotherapy and/or chemotherapy, and tumor stage and grade were similar between the VATS and open groups. However, a key issue with comparing the two surgical approaches did arise from the non-random case selection in the studies. Although the criteria varied slightly across different trials, patients were generally reported as eligible for VATS if their tumor was less than 5 cm (12,14,16,17) or 6 cm (13) in diameter. Furthermore, most studies required the tumor to be sufficiently separate from the innominate vein and other vital organs, including the great vessels, heart, and trachea, with no evidence of local invasion (9,11-16). As a result, tumor size was on average greater in the open thymectomy patients compared to VATS (5.6 vs. 4.5 cm, respectively). This difference may have resulted in selection bias and potentially skewed results towards more positive outcomes for the VATS group.
It should be noted that two of the included studies used propensity-matching to adjust for the possible confounding effect of tumor size (10) and additionally, Masaoka stage (9), and still demonstrated similar or superior disease-free survival in VATS compared to open thymectomy patients at 5 years. Furthermore, exceptions to the VATS selection criteria were reported. A small proportion of patients with tumors over 5 cm in diameter still underwent VATS in at least three of the studies (11,12,14). The potential feasibility and safety of the VATS approach for bulky intrathoracic benign lesions over 5 cm size was moreover demonstrated by Gossot et al. [2007], albeit in a single-arm (23). Agasthian [2011] further showed that VATS thymectomy could be performed for 13 invasive Masaoka stage III and IV thymomas <5 cm in size, with only one recurrence over a median follow-up of 4.9 years (24). Evidently however, adequately powered randomized controlled trials are required to confirm the efficacy of VATS thymectomy compared to open surgery for a broader range of thymic tumor types (22).
Variations in surgical techniques were also observed across the studies. A greater proportion of VATS patients received a ‘partial-’ or ‘hemi-’ thymectomy (20.8%, range, 0.0-59.2) compared to open surgery patients (1.5%, range, 0.0-36.4). Although partial thymectomy may raise concerns about tumor recurrence in the remaining thymic tissue, proponents argue that this is extremely rare and that an unnecessarily wide resection for an early-stage lesion in a non-myaesthenic patient may increase the risk of operative complications (11,25,26). Moreover, in the current review, disease-free survival was still similar or superior in VATS patients compared to open thymectomy patients, despite variations in resection extent. This result also eventuated despite a small percentage of the VATS group demonstrating positive resection margins (0.8%) (10,11,14,21). Another concern with partial thymectomy has been that of post-thymectomy myasthenia gravis (PTMG); however, this complication was insufficiently reported in the studies included and therefore not presented in our analysis. Nonetheless, PTMG, which has an estimated incidence of 1-3%, has been shown to occur even after cases of extended thymectomy, suggesting that full resection is not necessarily more protective compared to partial thymectomy (27). Future studies comparing different extents of resection for thymoma are required to determine their respective efficacy and safety.
Inter-study variations in the approach to open thymectomy were likewise observed. Although the majority of studies exclusively utilized a midline sternotomy (n=474) a small proportion of patients (n=47) in the trial by Yuan et al. [2014] underwent a thoracotomy instead (19). Subgroup analysis in this trial revealed similar outcomes for the sternotomy versus thoracotomy patients, with the exception of greater blood loss and longer operation times for a sternotomy. Thoracotomy, conversely, was shown to have shorter operating times than for VATS. When the two open techniques were combined in a comparative analysis against VATS thymectomy, operative times were subsequently similar, although blood loss and length of hospital stay remained greater for open thymectomy (19). Although these subgroup differences may have influenced the results of the current review, their effect is likely to be minimal given the relatively small proportion of patients who underwent a thoracotomy.
Other factors to consider include potential complications of VATS, which, although inconsistently reported across the studies, included phrenic nerve injury (6.7% in VATS vs. 0% in open). The risk of these complications may well be increased in the initial stages of the learning curve associated with VATS thymectomy (21,22,28). In addition, VATS has been suggested to increase the risk of pleural dissemination and recurrence. Proposed mechanisms for this increased risk have included excessive manipulation of the thymoma in the restricted working space of the anterior mediastinum, making the tumor capsule more prone to tearing, as well as incision of the mediastinal pleura, which may facilitate seeding of tumor cells (29). Although lower rates of recurrence for VATS compared to open thymectomy were demonstrated in the current review, longer follow-up to at least 5 years in future studies is required to confirm this demonstration of equal, or potentially superior, oncological efficacy.
Limitations
This review had several limitations inherent to the studies analyzed. These included their non-randomized, observational nature, small sample sizes, and insufficient and/or inconsistent reporting of outcomes including long-term survival, recurrence rates, and postoperative complications. There was a particular paucity of data reported after 5 years of follow-up, restricting the types of statistical comparisons that could be made. As pooled averages could not be calculated, meta-analysis was not performed. As previously discussed, variations in study protocols, including eligibility criteria for VATS and surgical techniques for VATS and open thymectomy, may have also contributed to selection bias and heterogeneity in results. Qualitative evaluation using MOOSE criteria further demonstrated an apparent paucity of independent assessment of outcome parameters by at least two investigators and specification of trial inclusion and exclusion criteria, based on lack of reporting by their respective studies.
Conclusions and recommendations
VATS thymectomy is emerging as an increasingly feasible and efficacious alternative to open surgery for resection of thymomas. The present systematic review reaffirmed several potential benefits of VATS compared to open thymectomy, which included similar, if not superior, overall and disease-free survival, reduced blood loss, lower rates of complications such as pneumonia, and shorter hospital stays. However, given the limitations inherent in retrospective observational studies with small sample sizes, further adequately powered trials with longer-term follow-up and future randomized controlled trials are required to confirm the comparative safety and efficacy of VATS thymectomy for thymoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [PubMed]
- Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2014;62:468-74. [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [PubMed]
- Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]
- Chao YK, Liu YH, Hsieh MJ, et al. Long-term outcomes after thoracoscopic resection of stage I and II thymoma: a propensity-matched study. Ann Surg Oncol 2015;22:1371-6. [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [PubMed]
- Chung JW, Kim HR, Kim DK, et al. Long-term results of thoracoscopic thymectomy for thymoma without myasthenia gravis. J Int Med Res 2012;40:1973-81. [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-1237.e1.
- Tagawa T, Yamasaki N, Tsuchiya T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [PubMed]
- He Z, Zhu Q, Wen W, et al. Surgical approaches for stage I and II thymoma-associated myasthenia gravis: feasibility of complete video-assisted thoracoscopic surgery (VATS) thymectomy in comparison with trans-sternal resection. J Biomed Res 2013;27:62-70. [PubMed]
- Yuan ZY, Cheng GY, Sun KL, et al. Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center. J Thorac Dis 2014;6:726-33. [PubMed]
- Cheng YJ. Videothoracoscopic resection of encapsulated thymic carcinoma: retrospective comparison of the results between thoracoscopy and open methods. Ann Surg Oncol 2008;15:2235-8. [PubMed]
- Maniscalco P, Tamburini N, Quarantotto F, et al. Long-term outcome for early stage thymoma: comparison between thoracoscopic and open approaches. Thorac Cardiovasc Surg 2015;63:201-5. [PubMed]
- Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [PubMed]
- Gossot D, Izquierdo RR, Girard P, et al. Thoracoscopic resection of bulky intrathoracic benign lesions. Eur J Cardiothorac Surg 2007;32:848-51. [PubMed]
- Agasthian T. Can invasive thymomas be resected by video-assisted thoracoscopic surgery? Asian Cardiovasc Thorac Ann 2011;19:225-7. [PubMed]
- Onuki T, Ishikawa S, Iguchi K, et al. Limited thymectomy for stage I or II thymomas. Lung Cancer 2010;68:460-5. [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [PubMed]
- Toker A, Erus S, Ozkan B, et al. Does a relationship exist between the number of thoracoscopic thymectomies performed and the learning curve for thoracoscopic resection of thymoma in patients with myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:152-5. [PubMed]
- Lucchi M, Davini F, Ricciardi R, et al. Management of pleural recurrence after curative resection of thymoma. J Thorac Cardiovasc Surg 2009;137:1185-9. [PubMed]





