Video-assisted thoracoscopic surgery for complex mediastinal mass resections
Introduction
Video-assisted thoracoscopic surgery (VATS) has an evolving role in the management of mediastinal pathology. Its use in diagnosing mediastinal masses is well established (1). While most surgeons still rely on a traditional open approach for larger, complex masses, VATS is increasingly being favored for small, benign mediastinal masses (2). However, the potential to use minimally invasive techniques for larger or more complex lesions exists. The mediastinum affords a narrow working space with vital organs and key structures that pose significant challenges to a VATS approach. However, as VATS techniques and instrumentation are further refined, successful minimally invasive resections of challenging mediastinal lesions are being increasingly reported. Masses such as teratomas, thymomas, and cysts noted for their size or involvement of surrounding structures are now amenable to safe thoracoscopic removal (3-5). The benefits of VATS in decreasing postoperative pain, morbidity, and length of hospitalization are well described for many procedures, as its therapeutic role in the management of mediastinal pathology continues to grow (6-9). Robotic surgery, a type of minimally invasive thoracic surgery, has advantages over VATS in that the instruments articulate within the chest and visibility is improved with the binocular camera. Possibly, greater progress in minimally invasive approaches to complex mediastinal lesions may be made first through robotic surgery given these advantages. The disadvantage of the robotic technique lies in the costs associated with its use. However, for those surgeons who perform the majority of their cases minimally invasively, their skill set is well suited to make advances with a VATS approach. With each new challenge comes “lessons learned”, which can be applied to additional procedures/pathology as encountered. One question remains: are minimally invasive approaches acceptable in the management of malignant mediastinal pathology? There is little objective evidence to suggest that they are not. Mounting evidence would suggest that oncologic outcomes for other site locations might be equivalent or improved with minimally invasive approaches for malignant disease (10,11). Similar approaches have been used to resect many other malignancies with reported equivalent outcomes, although follow-up is short (12). However, as time progresses, such data will become available. Besides the obvious, there may be additional benefits to a VATS approach. Given the magnified view with minimally invasive surgery, and the ability to view the thoracic cavity from multiple perspectives, we have identified drop metastases at the time of thymectomy for thymomas, which would have otherwise been missed with an open approach.
As surgeons continue to push the limits of VATS, the question becomes how does one effectively convey the education obtained through decades of experience performing such procedures to those in training or those interested in adopting a more minimally invasive approach. This keynote address will outline the general principles of VATS for the resection of complex mediastinal masses that we use, including operative planning, instrumentation, technical strategies, and lessons learned. This is not a representation of similar cases performed the same way, but adjustments in technique performed for diverse complicated resections over many years. We will first define what we mean by “complex” in the context of mediastinal pathology and then highlight specific strategies to optimize working with these variables. Later in this manuscript, critical teaching points will be highlighted by italicized key phrases that summarize particular strategies.
Defining “complex”
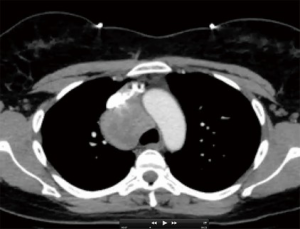
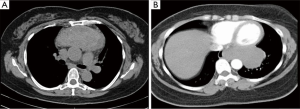
For the purposes of this address, “complex” is meant to include lesions whose resectability is complicated by one or more variables. Anatomic factors such as involvement or close proximity to nerves, airway, esophagus, great vessels, or heart are variables that some may consider to preclude the use of VATS reference. Additional anatomic variables may also include extremes of location within the chest. In particular, posterior mediastinal lesions may involve the extremes of the chest continuing into the thoracic outlet or retro-peritoneum (Figure 1).
The size of the lesion is also another variable that may contribute to complexity. For bulky lesions, working around these tumors poses unique challenges. As well, given the fixed space of the thoracic cavity, as lesions increase in size, the working space decreases. Although bulky mediastinal masses have proven to be amenable to thoracoscopic resection, these cumbersome masses have an added degree of surgical complexity (2) (Figure 2). Additional variables such as histology, benign versus malignant, re-operative surgery, patient body habitus, and tumor vascularity all pose significant challenges rendering operations more complex. Many of these cases typically involve more than one of these variables, and thus increase the challenge of the resection.
Common themes of operative planning
Technical aspects of resection can vary on a case-by-case basis; however, there are important themes common to any VATS approach. Thoughtful planning prior to the operating room is critical when considering minimally invasive resections of complex lesions. Unlike some surgical specialties, thoracic surgery varies in that there are often a number of potential approaches to an operation. This is true for open procedures, as well as for minimally invasive procedures. For those who may not have used a particular approach, orientation to the normal anatomy is a critical part of operative planning. We have found digital anatomy tools, specifically BioDigital Human (www.biodigitalhuman.com), to be useful. The perspective of the anatomy can be altered to mimic that found with a specific approach. For example, for the resection of an apical thoracic outlet schwannoma, the anatomy involved is complex. This digital model highlights some of the important anatomy (Figure 3A), while, this model highlights the anatomy only from the operative perspective (Figure 3B). We have found this to be a particularly useful tool at teaching conferences when reviewing the optimal minimally invasive approach. We find that, given the potential for different approaches, anatomical orientations may be particularly obvious to the mature thoracic surgeon but less apparent to our trainees or junior attendees.
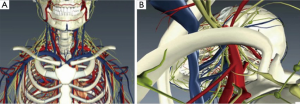
This resource allows for full three-dimensional (3D) visualization of relevant chest anatomy and may help in visualizing the trajectory of planned port placement. In addition, it also aids those who need assistance transitioning the orientation of the anatomy from what they know to what they do not. These models are limited in that they represent “normal” anatomy. Today, there are multiple free software programs that allow for the 3D reconstruction of individualized patient’s anatomy directly from their DICOM files. This is yet another tool to aid the surgeon in preoperative planning (www.OsiriX.com).
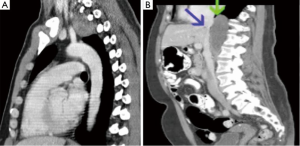
In addition, most thoracic surgeons tend to comprehend best when looking at computerized tomograms through axial slicing, as this is what we have done for the past several decades. However, our radiology department routinely provides sagittal and coronal images as well. Increasingly, these are helpful in the operative planning stage as well as intra-operatively, as a reference. When there is a question of invasion of a mass through the pericardium, additional imaging can be useful. Because of the motion, the cardiac border is not always delineated on CT and this motion artifact can be misinterpreted as invasion. Cardiac magnetic resonance imaging is very useful in ruling out attachment to the myocardium (Figure 4).

Patient positioning
Historically, thoracic surgery was performed in either the lateral decubitus or supine positions. Modifications to these positions were described but not widely utilized. As the complexity of VATS procedures has grown, so have the options for positioning. We currently think of positioning as degrees on a protractor, where supine is 0, lateral decubitus is 90 and prone is 180. For approaches to the anterior mediastinum we most commonly use a semi-supine position or about 15 degrees; for the middle mediastinum, including the apex of the chest, we use about 90 degrees; and for the posterior mediastinum, more towards 120 degrees (Figure 5).
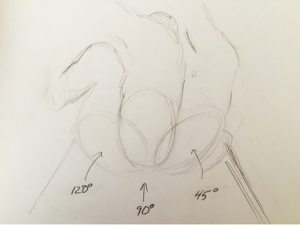
Optimal positioning needs to take several factors into consideration beyond access to the pathology, as for open approaches. For VATS, positioning needs to take into consideration not only the technical aspect of the dissection with the port placement options. Additional to this is the utility of gravity in aiding the procedure. For some approaches to the posterior mediastinum, prone positioning will allow the lung to fall away from the area of dissection. This frees up a port site that might otherwise be used to retract the lung. For other approaches, gravity has little impact. Although we have worked with patients in the prone position for some posterior mediastinal lesions, we find there is little advantage of the prone position over the semi-prone position. The latter has the advantage of the traction of gravity on the lung but one can more readily convert to open if needed. When positioning the patient in a semi supine position, the ipsilateral arm is brought posteriorly. One must insure that it does not interfere with the trajectory of the instruments (Figure 6).
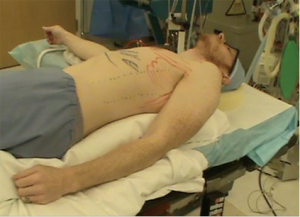
Surgeon position
We typically stand facing the patient’s front, when the patient is in lateral decubitus. As well, we teach with both the attending surgeon and the trainee on the same side. This has some advantages as no one is working against the orientation of the camera, and the attending can readily take over a portion of the dissection. Typically, trainees can watch an operation but not understand how to do the operation until they try for themselves. It is challenging for them to watch an experienced surgeon, who makes the dissection look simple, appreciate the exact technical maneuvers needed to safely complete the procedure. With both trainee and attending on the same side, the two can work back and forth, readily giving instruction or demonstrating technique as the operation progresses (Figure 7).
Instruments
We often use traditional laparoscopic instruments in addition to some of the more traditional VATS instruments. This is for several reasons. The laparoscopic instruments have some advantages: they come in 3 and 5 mm sizes, are typically smaller than traditional VATS instruments, they have been well designed for minimally invasive procedures, and work through sealed ports to maintain a pressure of carbon dioxide (CO2). However, they have disadvantages, such as not being curved. In particular, the lack of curvature is a disadvantage for chest wall work, and for adhesiolysis during re-operative surgery. Otherwise, these instruments work well in the mediastinum, as it is the central compartment and the trajectory is directly from the chest wall.
Laparoscopic instruments are small caliber and come in a variety of lengths, making them well-suited for mediastinal VATS approaches. Smaller caliber instruments are preferred in part because of the ease of port placement and potential traumatic injury to the intercostal nerves. If at any point during an operation, progress slows or it turns difficult, there should be no hesitancy to place an additional 3- or 5-mm working port, as it will aid in the exposure or dissection and these minor incisions are hardly noticeable to patients during their recovery. Trauma to the intercostal nerves with these small ports is typically minimal and does not contribute significantly to postoperative pain.
A 5-mm thoracoscope, angled 30 to 45 degrees, is used to facilitate visualization around bulky lesions and affords the best operative angles in the mediastinum. In expanding one’s practice in minimally invasive surgery, one should experiment with different camera angles to fully understand the benefits of these angles. This can be done on a routine uncomplicated resection, so that when one progresses to the a more complex mediastinal procedure, one is aware of how the different angles might assist you during a more challenging procedure.
For cautery, we typically use a combination of bi-polar and mono-polar. Mono-polar is quicker for pleural division and adhesiolysis, but we more often use bipolar electrocautery for its lower temperatures at the site of contact (14). Mediastinal lesions are typically in close proximity to one or several important nerves, and nerve injury from damaging heat diffusion can be problematic. The bipolar tissue sealing system results in minimal thermal spread in the tissues surrounding its sealing line and thus is well suited for VATS (15).
Port placement
Carefully thought out port placement cannot be overemphasized. Triangulation of ports and proper convergence of instruments is necessary for the dissection. We use a standard approach for the various compartments of the mediastinum. However, we often adjust port placement from time to time, during the operation, as the particular procedure dictates. For anterior mediastinal lesions, ports are placed along the infra-mammary fold and lateral crease along the anterior axillary line. As the course of the dissection proceeds, we will often change the trajectory of the ports, skipping an intercostal space in either direction to facilitate the dissection without traumatizing the intercostal nerve by torqueing on the ports around the rib. In addition, we have found a superior port positioned below the clavicle particularly useful for the superior mediastinum (16) (Figure 8A). For central mediastinal lesions, port placement is more traditional. When needed, in particular, for posterior mediastinal lesions, we often add a 4th port, typically 3 mm for additional retraction as the procedure dictates (Figure 8B).

Carbon dioxide (CO2)
CO2 insufflation set at 10 mmHg is used to improve exposure in most every case. Hesitancy to utilize CO2 insufflation in thoracoscopic surgery may stem from the concern that insufflation creates a pneumothorax and can lead to hemodynamic compromise. Low-pressure insufflation up to 10 mmHg can be safely used without negative impact on hemodynamics while greatly improving exposure and working space (17). For medial ports, in particular, on the left side, the CO2 keeps the heart away from interfering with the ports and instruments. The above considerations apply to all VATS cases but are especially important when approaching challenging resections. CO2 can also be used to aid in the evacuation of smoke from the chest cavity during the dissection.
As our experience has progressed, patients returning to the office following minimally invasive thoracic surgery, no longer complain of pain in their chest but instead pain in their throat from the double lumen tube. This has become the new frontier to address. With this, we have begun to routinely use a single lumen tube and CO2 insufflation for compression of the lung. This may be a concern for a number of reasons. Firstly, what happens if one crosses into the contralateral pleural space? Although theoretically this may be a problem, we have found after several entries into the contralateral pleural space, as it is almost never entirely avoided, that it is not a problem. Our anesthetist colleagues are able to increase the pressure to compensate, and oxygenation and ventilation of the patient has not been a problem. Second, when making a larger incision either sub-xyphoid or into the chest for extraction of larger tumors, the CO2 pressure is immediately released and one loses all visibility. Although this can happen, we have developed a strategy to avoid this problem. In this setting, prior to making the additional opening for extraction, we again work with our anesthetist colleagues. We have them make the patient apneic, thus avoiding re-expansion of the lung. These two techniques work well for the challenges that arise performing mediastinal dissection with a single lumen endotracheal tube.
Strategies to manage complicating factors
Size
Size of mediastinal lesions can be a challenging factor leading to their complexity. When dealing with lesions of large size, certain strategies aid the dissection of benign cysts and decompression may aid in their resection. However, if there is involvement of the esophagus, one may prefer to keep the contents of the cyst in order to help determine the wall of the cyst from esophageal mucosa.
For solid tumors, angled cameras allow one to work around the bulk of the tumor. Other options include dissection from both sides of the chest. For benign lesions, we will typically place the tumor in a sac and then break it up into smaller pieces where pathology may be assessed but margins will not. If there is concern for malignancy as in the case of thymoma, the mass should remain intact. The sub-xyphoid route works well for extraction for these larger lesions. There have been recent reports of sub-xyphoid VATS approach for anterior mediastinal dissections; however, we have little experience with this technique.
A special mention should be made about mediastinal goiters. When there is a cervical component, the intra-thoracic component can be dissected minimally invasively and delivered through the cervical incision. Although not all substernal goiters are amenable to this approach, we have found very few that are not. For completely ectopic goiters, we have performed the dissection thorascopically and then broken up the mass into smaller pieces for extraction (Figure 9). This allows for the tissue to be examined pathologically, but does not allow for margins. For these dissections, given the location, greater margins are not possible. As well, the likelihood of malignancy is low. Lastly, if thyroid cancer is found, patients receive appropriate therapy including radioactive iodine but more surgery for greater margins is not the recommendation nor is it possible without resection of vital structures, and for this pathology this does not make sense.
Although some consider size greater than 4 cm to be a contraindication for a minimally invasive approach to thymoma, this was originally based on experience with transcervical thymectomy. With VATS, several authors have reported successful resection of larger thymomas with a minimally invasive approach. We have also found this to be possible and that working with other larger mediastinal pathology has further enabled the use of similar approaches for larger thymomas (18).
Location
Location of the lesion to be resected can contribute to the complexity of the resection. This is typically the case when the pathology is in the mediastinum but at the extremes, or crosses into another cavity, as in thoracic outlet schwannomas or other pathologies bordering the inferior boundaries of the chest. For lesions in proximity to the hiatus, a laparoscopic transhiatal approach for epiphrenic diverticuli provides optimal visibility of the lower mediastinum (Figure 10). For more posterior lesions, we prefer to follow the insertion of the diaphragm. For posterior mediastinal lesions, increasing the tidal volume on the contralateral lung may aid in delivering the lesion into the ipsilateral chest and improve access to the lesion.
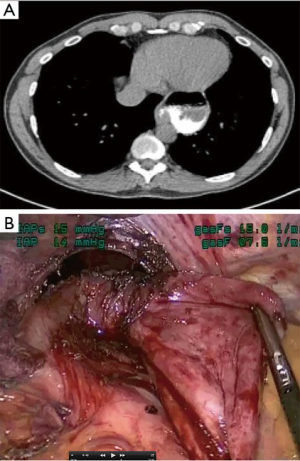
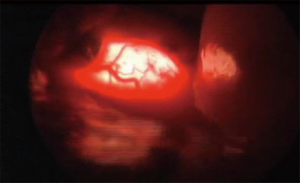
For lesions in close proximity to the esophagus, inadvertent injury to the mucosa can have devastating consequences. We have found two techniques particularly useful to aid in the identification of the esophagus and decrease the likelihood of injury. Placement of a bougie during the dissection gives the esophagus more substance and rigidity. This provides tactile feedback to the surgeon while working in the vicinity of the esophagus. For leiomyomas of the esophagus, the bougie can help define the extent of the leiomyoma. As these lesions can protrude into the lumen and potentially make mucosal injury more likely, an intra-luminal bougie will push the tumor away from the lumen, maintaining it. In addition, we routinely perform intra-operative esophagoscopy for lesions in close proximity to the esophagus. Trans-illumination may help identify a mucosal injury. This is done by turning off the light source on the thorascope, so that the light of the esophagogastroduodenoscopy (EGD) shines through (Figure 11). If there is concern for a leak, the esophagus can be covered with water and insufflation from the scope may demonstrate bubbles leaking from the esophagus.
Vascularity
Propensity to bleed is another variable that will increase the complexity of resecting mediastinal pathology. The most vascular tumors that we have operated on include mediastinal hemangioma, hyalinizing vascular Castleman’s and metastatic renal cell carcinoma. Of these, modern sealing devices as an alternative to traditional cautery are much better suited to control bleeding. We prefer the bi-polar type of device as there is less risk of injury to the nerves. With all of these resections, one must always keep the safety of the patient first and foremost. When needed, open techniques should be readily utilized. In addition, interestingly, we have not yet seen a chyle leak despite observing chyle leaking at the time of surgery. We have used these sealing devices to seal the lymphatics in addition to blood vessels, and although there is not much literature on the subject, we expect that these devices are just as effective at sealing lymphatic vessels as blood vessels. We do not empirically ligate the thoracic duct even when it has been fully dissected (Figure 12).
Post-operative drainage and pain control
As we have progressed, we have noticed that there appears to be little reason for a chest tube following a mediastinal resection for the majority of these cases. If the operation has gone smoothly and there is minimal bleeding, following the intercostal block, we will re-expand the lungs, and while a valsalva is being performed, remove the chest tube. Over the past several years we have progressed from leaving a chest tube in overnight, to removing it the night of surgery, to now removing it in the operating room for uncomplicated resections. We place one temporarily while the lung is being re-expanded. This requires aggressive support from our anesthesia colleagues to evacuate all of the CO2 and eliminate the atelectasis. We have found that this allows patients to sleep more comfortably overnight and they are ready for early discharge the next day.
Although the majority of patients are sent home on the first or second postoperative day, occasionally we have performed these resections as outpatient procedures. This is very much patient driven. In addition, we have an aggressive pain control regiment that starts in the operating room. Patients are given intravenous Tylenol and Toradol. At the completion of their procedure, and intercostal blocks are performed with liposomal bupivacaine (ExparelTM). This provides excellent analgesia for up to 96 hours and results in decreased narcotic use (senior author, unpublished data).
Lessons learned
VATS has progressed over the last two decades and more recently has seen its use increase in therapeutic management of mediastinal masses. Masses previously limited to open approaches are now being resected via VATS. The majority of thoracic surgeons are still performing open approaches, but adoption of minimally invasive techniques for the management of mediastinal pathology is progressively being more frequently reported. As we have seen in VATS wedge resection and lobectomy, the techniques for mediastinal resection will quickly progress as they are further refined and new knowledge gained. Although this group represents much different pathology, there are common principles for approaching complicated lesions that can be applied broadly. Careful review of the pathology and pertinent anatomy is a critical aspect for safety and success. Mental visualization of the orientation of the anatomy and potential challenges is critical for a safe operation. Optimal port placement can be a challenge, and if there is any doubt, it is best to place an initial port and then strategize accordingly. On that same note, frequent changes in trajectory and angles are optimal as an operation progresses and ports may be moved along several intercostal spaces during the procedure via the same skin incision to minimize nerve trauma. Flexibility in port placement is key for successful VATS. Although we typically use a 30 degree camera, on occasions the 45 or 75 degree might optimize the view. If one has not, changing cameras during a straightforward operation may be useful in becoming familiar with the advantages and disadvantages of the variable angles. Postoperative care has also dramatically changed as a result of advances in instrumentation and technology. Advances in pain control have helped expedite discharges and reduce patient stays from several days to observation and occasionally same day procedures. Although specific steps like positioning, port placement, and CO2 insufflation should be followed, the key to these resections is adaptability and strategic problem solving. Surgeons should not hesitate to change their approach, port placement, and camera angles once dissection changes from easy to difficult while constantly considering the critical structures, often hidden from view, at each junction of dissection. Typically we begin each resection with the easiest part, until it becomes difficult. At the point, rather than persist, move to a different part of the dissection, until that too becomes difficult. Proceeding in this fashion one continues to make progress, avoids injury, surgeon fatigue, and frustration. These all contribute to the safety of the procedure. There are no strict rules to follow although there is one additional critical technical aspect of these procedures. Because the camera provides a magnified view, it is important to periodically pull the scope back and take a panoramic view of the chest to re-orient oneself to the anatomic perspective. It is very easy to become disoriented during the dissection and lose anatomic perspective.
Given the advances in VATS over the past several years there is little doubt that its use will continue to broaden and become the standard approach for most intra-thoracic lesions, including mediastinal masses. We look forward to the innovative instrumentation and techniques that will allow thoracic surgeons to perform progressively less invasive procedures and improve surgical outcomes for our patients while minimizing tissue trauma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Roviaro G, Varoli F, Nucca O, et al. Videothoracoscopic approach to primary mediastinal pathology. Chest 2000;117:1179-83. [PubMed]
- Gossot D, Izquierdo RR, Girard P, et al. Thoracoscopic resection of bulky intrathoracic benign lesions. Eur J Cardiothorac Surg 2007;32:848-51. [PubMed]
- Rothermel L, Gilkeson R, Markowitz AH, et al. Thoracoscopic resection of a giant teratoma compressing the right heart. Interact Cardiovasc Thorac Surg 2013;17:594-7. [PubMed]
- Vyas S, Agasthian T, Goh MH, et al. Thoracoscopic thymectomy in a previous sternotomy. Asian Cardiovasc Thorac Ann 2006;14:e108-10. [PubMed]
- Lazopoulos G, Pavlopoulos D, Kambitakis E, et al. Huge cystic lymphangioma of the mediastinum successfully treated with thoracoscopic surgery. Ann Thorac Surg 2014;98:2233. [PubMed]
- Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 1993;56:1285-9. [PubMed]
- Landreneau RJ, Wiechmann RJ, Hazelrigg SR, et al. Effect of minimally invasive thoracic surgical approaches on acute and chronic postoperative pain. Chest Surg Clin N Am 1998;8:891-906. [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [PubMed]
- Nwogu CE, D'Cunha J, Pang H, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). Ann Thorac Surg 2015;99:399-405. [PubMed]
- Midura EF, Hanseman DJ, Hoehn RS, et al. The effect of surgical approach on short-term oncologic outcomes in rectal cancer surgery. Surgery 2015;158:453-9. [PubMed]
- Adam MA, Choudhury K, Goffredo P, et al. Minimally Invasive Distal Pancreatectomy for Cancer: Short-Term Oncologic Outcomes in 1,733 Patients. World J Surg 2015;39:2564-72. [PubMed]
- Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2013;96:951-60. [PubMed]
- Marshall MB, DeMarchi L, Emerson DA, et al. Video-assisted thoracoscopic surgery for complex mediastinal mass resections. Asvide 2015;2:143. Available online: http://www.asvide.com/articles/720
- Santini M, Vicidomini G, Baldi A, et al. Use of an electrothermal bipolar tissue sealing system in lung surgery. Eur J Cardiothorac Surg 2006;29:226-30. [PubMed]
- Pons F, Lang-Lazdunski L, Bonnet PM, et al. Videothoracoscopic resection of neurogenic tumors of the superior sulcus using the harmonic scalpel. Ann Thorac Surg 2003;75:602-4. [PubMed]
- Marshall MB. Thorascopic mediastinal resection after median sternotomy and mediastinotomy. Ann Thorac Surg. 2009;88:1371-3. [PubMed]
- Ohtsuka T, Imanaka K, Endoh M, et al. Hemodynamic effects of carbon dioxide insufflation under single-lung ventilation during thoracoscopy. Ann Thorac Surg 1999;68:29-32; discussion 32-3. [PubMed]
- Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [PubMed]