Multi-institutional European experience of robotic thymectomy for thymoma
Introduction
Radical thymectomy is the gold standard treatment for resectable thymomas, with completeness of resection representing the most important prognostic factor (1). Currently, median sternotomy is widely considered as the standard approach for thymoma resection at any stage, allowing a technically easy and oncologically safe operation (2). While the video-assisted thoracoscopic surgery (VATS) approach has been extensively used for mediastinal diseases in the last three decades, it was mainly confined to the treatment of several benign diseases or to thymectomy in cases of non-thymomatous myasthenia gravis (MG) (3-5). The first VATS approach for thymoma was described in early 1990s (6); since then, only few authors have published small series of VATS thymoma resections with short-term follow-up, leading to a paucity of clear and sound data about the effectiveness of this approach (2,7-9). Consequently, a number of surgeons are still reluctant to use this surgical approach that remains controversial—the supposed increased risk of local recurrence (due to reduced safety margins after minimally invasive resection) and the possible rupture of the capsule with implantation of the tumor during endoscopic manipulations are the most common arguments against the VATS approach. Furthermore, the lack of long-term oncologic results, the learning curve required to perform this operation safely and the relative rarity of this tumor are additional reasons that slow the adoption of the VATS resection for early stage thymomas (10). The introduction of robotic-assisted technologies in the late 1990s provided a technical advancement able to overcome the limitations of conventional thoracoscopy. Specifically, the three-dimensional vision system and the articulated instruments of the da Vinci Surgical Robotic System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) allow for an intuitive, ‘open-like’ intervention, but with minimally invasive access. The application of robotic technology has been tested in a variety of thoracic surgery procedures, particularly for mediastinal diseases, where the robotic system is thought to provide the maximum benefit (11,12). The aim of this study was to evaluate the safety and the feasibility of robotic thymectomy, analysing the oncologic outcome in a group of patients with clinically defined early-stage thymoma, in four European Centres with extensive experience in this type of operation.
Materials and methods
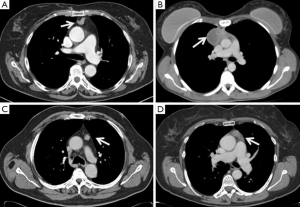
We reviewed the data of 134 patients undergoing robotic thymectomy for clinically defined early-stage thymoma (Masaoka stages I and II) collected between 2002 and 2014 by four European Thoracic Surgery Centres (University of Maastricht-Nederland; University of Padova-Italy; University of Pisa-Italy; University of Innsbruck-Austria). All patients signed a detailed consent form in which they were informed about possible complications of a thymoma resection with robotic approach and the lack of long-term data. The institutional review board of each centre approved the study. Information on patient demographics, presence of associated MG, tumor characteristics, stage, intra and postoperative data (e.g., complications, need for open conversion or additional ports or accesses, operative time, length of hospital stay) were collected. The Masaoka staging system was used to assess the pathological stage (2), while the new World Health Organization classification was used for histological definition (13). The Myasthenia Gravis Foundation of America (MGFA) classification (14) was applied to stratify the preoperative class of MG. Preoperative assessments included evaluation of pulmonary and cardiac functions, total body computed tomography (CT) or magnetic resonance imaging (MRI). Preferred radiological characteristics to be eligible for robotic thymectomy were the location of the tumor in the anterior mediastinum, a distinct fat plane between the tumor and surrounding structures, unilateral tumor predominance, tumor encapsulation, existence of residual normal appearing thymic tissue, and no mass compression effect (Figure 1) (7). In cases of unexpected intraoperative finding of involvement of surrounding structures (Masaoka stage III), pleuro-pericardial or pulmonary nodules (Masaoka stage IVa/b), the robotic approach was converted to an open approach if the resection was considered technically difficult, unfeasible or unsafe for the patient. Patients were followed up until death or May 2015, if alive, by periodic visits (with neurologists if affected by MG) and phone contact. A total body computed tomography scan was performed every six months for the first two years postoperatively, then every year. There were 61 (45.5%) males and 73 (54.5%) females, with a median age of 59 years (range, 14–88 years). Seventy (52.2%) patients were affected by MG.
Surgical technique
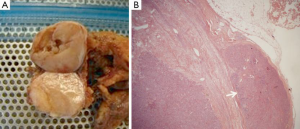
The side of surgical access was based on a single surgeon’s experience, or occasionally on the presence of unilateral tumor predominance. The surgical technique of robotic thymectomy from either the left or right side has been described in existing literature (15,16). This procedure was performed differently from thymectomy for non-thymomatous patients, with all surgeons adopting a “no-touch technique” for an “en bloc” resection of thymus and perithymic fat tissue. In this technique, the thymoma was never touched and the normal thymic tissue and peri-thymic fat were used for grasping and for traction. This technique avoids a direct manipulation of the tumor, in order to minimize the risk of tumor seeding in consequence of capsule damage. All thymus and perithymic fat were dissected with safe surgical margins, according to the International Thymic Malignancy Interest Group criteria (17), and the completeness of thymectomy was assessed by macroscopic inspection of the thymic bed, specimen and subsequent pathological analysis (Figure 2).
Statistical analysis
Data were expressed as absolute numbers, percentage, median or mean values ± standard deviation (SD). Survival curves were calculated by Kaplan-Meier method.
Results
The robotic approach was left-sided in 51 (38%), right-sided in 80 (59.8%) and bilateral in three (2.2%) patients. The median operative time was 140 minutes, ranging between 60 and 353 minutes (mean 146.4±43 minutes). Twelve (8.9%) patients needed conversion to an open approach (in two cases due to large diameter of tumor interfering with a safe dissection, in 10 cases for unexpected invasion of surrounding structures as the great vessels, lung or pleura-pericardial implants). In one (0.7%) case, a standard thoracoscopy was used after robotic system breakdown. In six (4.4%) cases, an additional access (cervicotomy in one case, an additional homolateral thoracoscopic port for suction purpose in five cases) was required. No vascular and nerve injuries were recorded, and no perioperative mortality occurred. A total of 23 (17.1%) patients had postoperative complications: four cases of atrial fibrillation, three cases of myasthenic crisis, three pneumothoraces after chest tube removal, three pleural effusions, two cases of pneumonia, one haemothorax treated conservatively by blood transfusion, one chylothorax, one orthostatic hypotension, one wound infection, one urinary tract infection treated with medical therapy, one pulmonary embolism, one mediastinal infection, and one pulmonary herniation. Median hospital stay was 4 days (range, 2–35 days; mean 4.8±2.5 days). Mean diameter of the resected tumors was 4.4±1.3 cm (range, 1–10 cm), Masaoka stage was I in 46 (34.4%), II in 71 (52.9%), III in 11 (8.3%), IVa in 5 (3.7%) and IVb in 1 (0.7%) patient. Histologic evaluation revealed 22 (16.4%) type A, 23 (17.2%) type AB, 23 (17.2%) type B1, 40 (29.8%) type B2, and 26 (19.4%) type B3 thymomas. At the last follow up (May 2015: median 42 months, range, 5–159 months; mean 48±35.7 months), 131 (97.8%) patients were alive, 3 (2.2%) patients died all for non-thymoma related causes (leukemia, vulval carcinoma and colon carcinoma). A pleural recurrence was found in 1 (0.7%) patient with original Masaoka stage IVa. The five-year overall survival rates were 97%, and the five-year thymoma-related survival rates were 100%.
Discussion
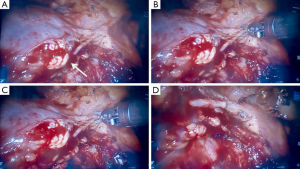
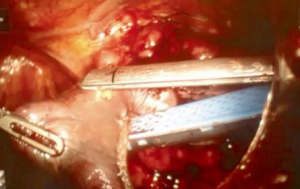
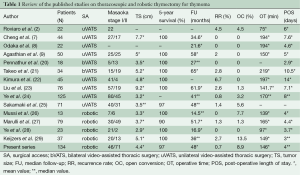
Since its introduction into clinical practice in the early 1990s, VATS has gained broad acceptance for diagnostic and therapeutic interventions for both pulmonary and mediastinal benign diseases (3-6). The main recognized advantages of VATS compared with open approaches are minimal operative trauma, lower morbidity, early improved pulmonary function, shorter hospital stays and better cosmetic results (3,4,18). These obvious advantages have increased the acceptability of the VATS approach, especially among patients with MG, leading to an increased number of thoracoscopic thymectomies being performed for non-thymomatous MG with good surgical and neurological results (15,16,19). Contrary to the lung cancer experience however, in which VATS resection has become the standard approach for early-stage NSCLC, most surgeons are still reluctant to perform a thoracoscopic thymectomy in patients with thymoma, due to technical and oncological concerns. There are a number of technical reasons to not use thoracoscopy: the upper mediastinum is a delicate and difficult-to-reach anatomical area with vulnerable large vessels and nerves, particularly with thoracoscopy. In the two-dimensional view of the operative field, the surgeon’s tremor is enhanced by the thoracoscopic instruments and they do not articulate, making it difficult to operate in a fixed three-dimensional space such as the mediastinum. Moreover, thoracoscopic thymectomy is considered a technically challenging operation with a steep learning curve (10). The oncological concerns relate to the possible breach of tumor capsule with risk of tumor seeding locally or in the pleural cavity, and the difficult evaluation of resection margins with reduced oncological accuracy and safety. The robotic surgical system has provided several advantages able to overcome some technical and methodological limits of conventional thoracoscopy: (I) the improved dexterity of instruments (7 degrees of freedom articulation, 360 degrees of rotation) allows complex three-dimensional movements, providing a safe and comfortable dissection around vessels, nerves, and tiny and remote areas such as the superior horns or the contralateral mediastinum; (II) the high-resolution, three-dimensional vision permits the best possible and magnified view of the surgical field; and (III) the filtering of hand tremors allows greater technical precision. In our opinion, these characteristics have significantly increased the safety and the oncological effectiveness of robotic thymectomy for thymoma. In fact, there is less manipulation of the thymic and perithymic tissue during the operation, and a better evaluation of healthy tissue as a result of the high quality image. This allows for a more precise and low-risk dissection with wide safety margins, and reduced possibility of an incautious tumor breaching, incomplete resection or iatrogenic injury. The lack of tactile feedback could theoretically increase the risk of damaging tumor capsule; however, this disadvantage seems widely compensated by the superior three-dimensional vision control of the system. In the last 15 years, several authors have published the results of thoracoscopic and robotic thymectomy for early-stage thymoma (Table 1). The available data confirm that this approach may be considered technically sound and safe in the hands of appropriately-trained surgeons. However, data are still inconclusive with regard to oncological outcome due to the lack of long-term follow-up. In fact, thymomas are indolent tumors, and a long lapse of time (at least 10 years) is necessary to evaluate the survival and relapse rate. Therefore, as pointed out by Davenport et al. in a systematic review (30), there is a lack of evidence in the current literature supporting a minimally invasive approach compared to a standard transsternal approach. At that time, the open transsternal surgical approach is widely considered the gold standard for resection of thymoma, ensuring the best chance for a complete resection (1,2). However, despite the lack of long oncological follow-up, the surgical results are outstanding: no major complications or mortality occurred in this large series. Other authors adopting either the conventional VATS or robotic approach also reported similar results (2,3,7-9,20,22-29). In contrast to other authors supporting a thoracoscopic subtotal thymectomy for non-invasive thymoma without MG as the preferred resection modality regardless of tumor size and tumor capsule characteristics (2,8-19,30,31), our policy was to undertake an extended thymectomy in all cases, such as in the open approach. In the absence of definitive long-term data, a standardization of the technique is necessary in order to avoid biases in the evaluation of the outcome. Moreover, we consider the intraoperative manipulation of the specimen to be safer when the perithymic fat tissue is contextually resected ‘en bloc’. Most of our patients (87.3%) had an early-stage tumor due to the selection criteria we adopted, based on the radiological criteria proposed by Cheng et al. (7): the location in the anterior mediastinum, tumor encapsulation, a distinct fat plane between the thymoma and vital organs, the existence of residual normal appearing thymic tissue, no mass compression effect and unilateral tumor predominance, particularly for tumors of dimension greater than 3 cm. However, while most cases were clinically diagnosed as Masaoka stage I, 52.9% patients were found to be Masaoka stage II, 8.3% were in stage III and 4.4% in stage IVa/b after resection and final histological evaluation. A similar finding was reported by Takeo et al. (21), where it was revealed that 57% of patients had Masaoka stage II and III after an initial clinical diagnosis of stage I, while in a report by Quintanilla-Martinez et al. (32), 28.5% of the tumor reported by the surgeon to be encapsulated showed a microscopic evidence of capsular invasion. In regards to Masaoka stage III and IV discovered at surgical exploration, our policy was to convert to an open access (sternotomy or thoracotomy) based on individual surgeon’s judgement. In particular, when the resection was considered unsafe or unfeasible by robotic approach, an open resection was performed; this occurred in 10 cases, while in the remaining seven cases, a resection extending to the pericardium, the phrenic nerve (Figure 3), lung (Figure 4) or parietal pleura were performed entirely by robotics. Despite being technically feasible, extended resections should be considered experimental and reserved to very select cases, as the oncological safety is still unknown. Another debated point is the appropriate size of thymoma for VATS or robotic resection; the majority of authors dealt with lesions smaller than 5 cm, but an average tumor diameter around 3 cm is generally considered as oncologically acceptable (10,27). In our experience, the mean diameter of resected lesions was 4.4 cm, with a range between 1 and 10 cm. A large tumor size was not considered an absolute contraindication; however it may interfere with the surgical procedure, making the manipulation more difficult with increased chance of an open conversion, prolonged operative time or capsule injury, as reported by Kimura et al. (22). In regards to the surgical results, no mortality, low morbidity and short hospital stay were observed. The operative times and open conversion rate were comparable with other series of thoracoscopic thymoma resection (Table 1). It is interesting to note that no conversions due to intraoperative vascular accidents were required, as the accurate vision allowed the surgeons to perform an optimal vascular dissection or identify early vascular invasion, avoiding any intraoperative damage. Looking at the oncologic outcome, a recurrence rate ranging between 0% and 6.7% has been reported in previous thoracoscopic and robotic series. In our experience, the single pleural relapse was observed in a Masaoka stage IVa despite a macroscopic radical intervention. Relapses also frequently occur in open surgery due to microscopic residual disease. Cheng et al. (33) and Pennathur et al. (20) compared the VATS and transsternal approaches for thymoma in small series, reporting no significant difference in recurrence rate and overall survival between the two groups. Although very encouraging, the oncological results need definitive validation, since the indolent nature of thymoma requires more mature data from longer follow-up. The present study has some limitations, particularly the non-randomized, retrospective and multi-institutional methodology. In addition, the follow-up period is still inadequate to allow a definitive conclusion on the oncological outcome.
Full table
In summary, robotic thymectomy for early stage thymoma is a technically safe and effective operation. In addition to the advantages of a minimally invasive approach (short hospital length of stay, excellent cosmetic results, low morbidity), increased visualization and instrument dexterity enabled by robotic technology provides further benefit compared to conventional thoracoscopy. Our data on a large number of patients are encouraging, particularly for early stage thymoma, despite a relatively short oncologic follow-up period. Extended resections for Masaoka stage III/IV may be possible for selected patients, but they are considered experimental.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [PubMed]
- Roviaro G, Varoli F, Nucca O, et al. Videothoracoscopic approach to primary mediastinal pathology. Chest 2000;117:1179-83. [PubMed]
- Yim AP, Kay RL, Ho JK. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [PubMed]
- Yim AP. Video-assisted thoracoscopic resection of anterior mediastinal masses. Int Surg 1996;81:350-3. [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [PubMed]
- Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192-6. [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [PubMed]
- Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann 2010;18:234-9. [PubMed]
- Toker A, Erus S, Ozkan B, et al. Does a relationship exist between the number of thoracoscopic thymectomies performed and the learning curve for thoracoscopic resection of thymoma in patients with myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:152-5. [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [PubMed]
- Savitt MA, Gao G, Furnary AP, et al. Application of robotic-assisted techniques to the surgical evaluation and treatment of the anterior mediastinum. Ann Thorac Surg 2005;79:450-5; discussion 455. [PubMed]
- Rosai J, Sobin LH, World Health Organization. Histological typing of tumours of the thymus. 2nd ed. Berlin; New York: Springer, 1999.
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Experience with the "da Vinci" robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [PubMed]
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [PubMed]
- Takeo S, Tsukamoto S, Kawano D, et al. Outcome of an original video-assisted thoracoscopic extended thymectomy for thymoma. Ann Thorac Surg 2011;92:2000-5. [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7.e1.
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6; discussion e47.
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [PubMed]
- Ye B, Li W, Ge XX, et al. Surgical treatment of early-stage thymomas: robot-assisted thoracoscopic surgery versus transsternal thymectomy. Surg Endosc 2014;28:122-6. [PubMed]
- Keijzers M, Dingemans AM, Blaauwgeers H, et al. 8 years' experience with robotic thymectomy for thymomas. Surg Endosc 2014;28:1202-8. [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [PubMed]
- Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymomas. Surg Endosc 2008;22:1272-7. [PubMed]
- Quintanilla-Martinez L, Wilkins EW Jr, Ferry JA, et al. Thymoma--morphologic subclassification correlates with invasiveness and immunohistologic features: a study of 122 cases. Hum Pathol 1993;24:958-69. [PubMed]
- Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest 2005;128:3010-2. [PubMed]





