Long term compensatory sweating results after sympathectomy for palmar and axillary hyperhidrosis
Introduction
Endoscopic thoracic sympathectomy is currently the treatment of choice for primary upper extremity hyperhidrosis (1). Medical treatments, such as local antiperspirants, systemic anticholinergic agents, iontophoresis and botulinum toxin, alleviate symptoms only transiently (2,3). Sympathectomy involves the interruption of the upper thoracic sympathetic chain through cauterization (electrocoagulation, ultrasonic scalpel, radiofrequency dissector) or clipping. Satisfactory results are reported all over the world after video-assisted thoracoscopic sympathectomy that was proved to be safe and effective (4,5). However, the potential for adverse effects, particularly the development of compensatory sweating, is a concern and often precludes surgery as a definitive therapy (6).
To date, amongst all the different surgical approaches, post-surgical compensatory sweating varies widely, ranging from 3% to 98% (2). Nevertheless, only few patients consider compensatory sweating to be as bothersome as their original hyperhidrosis symptoms (6,7), corroborating the high level of patient satisfaction after sympathectomy.
In fact, long-term results with regard to compensatory sweating and its evolution during the time have never been analyzed.
The present retrospective study aims to show long-term results comparing two-stage unilateral versus one-stage bilateral thoracoscopic sympathectomy.
Methods
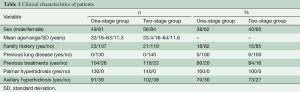
Between November 1995 and February 2011, 270 patients with severe palmar or palmar and axillary hyperhidrosis underwent to endoscopic thoracic sympathectomy (Table 1). All patients had excessive sweating in hands or hands and armpits, severely interfering with their work or social activities. One hundred and thirty patients received one-stage bilateral single port video-assisted thoracoscopic sympathectomy (one-stage group) and 140 patients two-stage unilateral single-port video-assisted thoracoscopic sympathectomy (two-stage group). The clinical characteristics of both groups are listed in Table 1. Before surgery, all patients underwent a careful clinical history, pre-operative routine blood examination, spirometry, cardiologic consulting and chest X-ray to exclude pulmonary disease. Written informed consent from all patients was obtained.
Full table
Long-term post-operative results, including residual pain, bradycardia, facial flushing or hyperthermia, compensatory sweating and recurrence of symptoms were analyzed. Each patient was evaluated at one and three years after surgery through a clinical examination and a questionnaire administration. The mean postoperative follow-up period was 7.2 years (range, 4–9 years). Potential variables responsible for compensatory sweating evolution were evaluated in both groups.
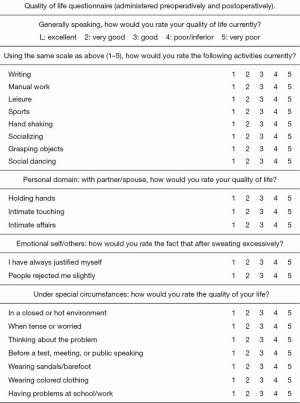
Finally, quality of life (QoL) was assessed administering a standardized study questionnaire used by Cerfolio et al. (6,7) and suggested by the Society of Thoracic Surgeons Expert Consensus for the Surgical Treatment of Hyperhidrosis (6). The questionnaire, shown in Figure 1, evaluates 20 items divided into four domains (functional, personal, emotional, under special circumstances domains), rating the QoL from one (excellent) to five (very poor). The minimum score [20] indicates an excellent QoL and the maximum score [100] indicates a very poor QoL. QoL was assessed before, at one and three years after surgery.
Surgical technique
Surgery was performed under general anesthesia. Double-lumen endotracheal intubation and selective one lung ventilation was used. Patients were placed on the operating table in a semi-sitting position with arms abducted more than 90 degrees. Only one incision of about 8-mm was performed in the third intercostal space on the anterior axillary line. In the one-stage group, the same body position was used to perform the procedure on the other side.
Three mm, 30° thoracoscope (Karl Storz, Tuttlingen, Germany) and 5 mm endoscopic dissector (B. Braun, Melsungen, Germany) were introduced into the thoracic cavity after lung exclusion on the operative side of surgery. The sympathetic chain was identified counting the ribs from the first rib. By opening the parietal pleura the sympathetic chain was exposed, identifying the T2-T4 tract. Dissection was performed by electrocautery from the second to the fourth ganglia. The thoracoscope and endoscopic instruments were then removed. A temporary 10Ch chest tube was inserted into the thoracic cavity through the surgical incision and connected to a water seal system applying a mild suction. After re-inflating the lungs, the chest tube was quickly removed and the incision was closed.
Chest X-ray was performed during the first postoperative day before the discharge.
In the two-stage group, the surgical procedure was performed on one side and undertaken on the other side after a mean time interval of 4 months (range, 1–12 months) from the contralateral procedure.
Statistical analysis
Values are reported as means ± standard deviation. Comparisons between one-stage and two-stage group data were analyzed using t-test and Wald chi-square test. Compensatory sweating evolution was evaluated using a Kaplan-Meye analysis. Potential variables other than one- or two-stage procedure responsible for compensatory sweating improvement, including age (≤30/≥31 years), gender, smoking status and palmar or palmar and axillary hyperhidrosis, were evaluated by Cox regression model. The hazard ratio and corresponding 95% confidence intervals were reported for covariates considered clinically relevant or statistically significant at the 0.05 level.
Results
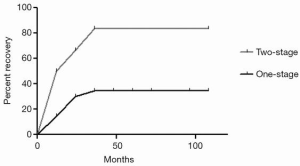
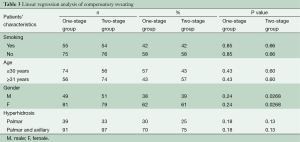
A total of 261 patients underwent video-assisted thoracoscopic sympathectomies (Table 2). All patients had an immediate result after surgery, having warm and dry hands, and showing full satisfaction. Histological analysis confirmed a normal nervous tissue. The mean postoperative follow-up period was 7.2 years (range, 4–9 years). Within 7 days after operation, in the one-stage group 16 patients (12%) suffered from mild/moderate pain, requiring more analgesics (morphinics or local analgesia with naropin infiltration) than the standard doses (90 mg of Ketorolac), versus 15 patients (11%) in the two-stage group. However, no patients showed a chronic pain or experienced Horner’s syndrome. Sixteen patients (12.7%) of one-stage group and 15 patients (11.1%) of two-stage group suffered from bradycardia (P=0.15). Recurrence occurred in three patients (2.4%) in the one-stage group and one (0.7%) in the two-stage group (P=0.09). Facial flushing or hyperthermia occurred in eight patients (6.3%) in the one-stage group and in 11 (8.1%) in the two-stage group. Compensatory sweating occurred in 27 patients (21.4%) in the one-stage group and 6 (4.4%) in the two-stage group (P=0.0001) with no strong association between all the independent variables evaluated (smoking, age, gender, palmar and palmar/axillary hyperhidrosis) at the multivariate analysis (Table 3). However, compensatory sweating recovered in five patients (83.3%) in the two-stage group versus 9 (33.35%) in the one-stage group during follow-up (Log-rank test P=0.016; HR, 7.196; 95% CI, 1.431–36.20, Figure 2). Cox regression model of recovery in patients who experienced compensatory sweating showed no association between all the independent variables evaluated (age, sex, palmar/palmar and axillary hyperhidrosis).

Full table
Full table
An improvement in postoperative QoL scores was observed in at least 90% of patients at one year after surgery in the one-stage group (36±12 versus 92±11, P=0.001) and at least 95% of patients in the two-stage group (34±16 versus 97±13, P=0.001) and at three years in the one-stage group (36±12 versus 93±17, P=0.001) and two-stage group (34±16 versus 98±15, P=0.001).
Discussion
In this report, we showed that both one-stage bilateral and two-stage unilateral video-assisted thoracoscopic sympathectomy for palmar and axillary hyperhidrosis are successful procedures with minimal invasiveness and few postoperative complications.
In our series, target resolution of the disorder was achieved in 100% of the patients. No recurrence was observed during the first follow-up points. The long-term recurrence rate was n=3 (2.4%) in one-stage group and n=1 (0.7%) in two-stage group (P=0.09). Facial flushing or hyperthermia occurred in eight patients (6.3%) in the one-stage group and in 11 (8.1%) in the two-stage group. Postoperative pain lasting less than one week was observed in 11–12% of our cases: only a few patients required opioids or local analgesia with naropin infiltration in addition to the standard doses of analgesics. There was no significant relevance for constant residual pain after seven days postoperatively. No patients demonstrated chronic pain. Sixteen patients (12.7%) in the one-stage group and 15 patients (11.1%) in the two-stage group experienced bradycardia with range values of 44–59 bpm and a mean duration time of 5.7 years for one-stage group and 5.9 for two-stage group (P=0.15).
Compensatory sweating occurred in 27 patients (21.4%) in the one-stage group and 6 (4.4%) in the two-stage group (P=0.0001). However, compensatory sweating recovered in five patients (83.3%) in the two-stage group and nine (33.35%) in one-stage group during follow-up (Log-rank test P=0.016; HR, 7.196; 95% CI, 1.431–36.20). Cox regression model of recovery in patients who experienced compensatory sweating showed no association between all the independent variables evaluated (age, sex, palmar/palmar and axillary hyperhidrosis).
Compensatory hyperhidrosis (postoperative increase of sweating in regions of the body where it had not been previously observed) is the most common late complication, with different incidence reported in previous studies, ranging from 33% to 85% (8,9), regardless of the number of ganglia removed (10), and showing a gradually decreasing intensity over the follow-up period. However, the mechanism of compensatory hyperhidrosis is still unclear. According to findings reported in literature, compensatory sweating seems dependent upon the height of the sympathetic chain resection. Compensatory sweating is greater with T2-T4 resection than with T2 or T3 only or T2-T3 resection (11). The published results do not provide a consensus regarding the most favorable technique, although treatment of axillary hyperhidrosis requires resection as far as T4 (12). Indeed, this negative repercussion motivates patients to avoid surgery (6).
Previous studies from our group showed that we showed that compensatory sweating is more frequent in the one-stage group (19% versus 4%, P<0.0001) (13). Thus, since experience with reversal is still fairly limited, we were interested in investigating the potential evolution of the compensatory hyperhidrosis. By previous reports, the improvement of this post-operative complication seems to unrelated to smoking habits according to the multivariate analysis (14,15). We speculated that after providing at least one month of time to the rami communicantes and the accessory fibers of Kuntz to regenerate, undergoing to a contralateral sympathectomy could provide a definitive thermoregulatory balance (16). Specifically, we are providing for the first time long-term results on compensatory sweating evolution, the main side effect after this surgical procedure. We investigated whether variables such as group age, sex, palmar/palmar and axillary hyperhidrosis could impact on compensatory sweating recovery. We found that recovery from compensatory sweating is affected by when sympathectomy is performed with a time interval between the two procedures. Patients seem to improve their condition experiencing lower rates of compensatory sweating during the time if they underwent a two-stage sympathectomy (HR, 7.196; P=0.016). Accordingly, the QoL long-term results improved after one-stage and two-stage VATS sympathectomy. It is noteworthy to mention that 8% and 3% of patients in the one- and two- stage groups, respectively, did not report improved QoL due to compensatory sweating. In contrast, 13% and 1% of patients who experienced mild/moderate compensatory sweating significantly improved their QoL. No differences in terms of QoL were observed at three years after surgical treatment.
In conclusion, our results suggest that two-stage unilateral video-assisted thoracoscopic sympathectomy allows better results to be achieved in terms of side effects and QoL. This approach should be considered in clinical practice.
Acknowledgements
The authors acknowledge Dr. M. Silvi, Data Manager, Division of Thoracic Surgery, Sant’Andrea Hospital, Faculty of Medicine and Psychology, University of Rome “La Sapienza”.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Walling HW, Swick BL. Treatment options for hyperhidrosis. Am J Clin Dermatol 2011;12:285-95. [PubMed]
- Li X, Chen R, Tu YR, et al. Epidemiological survey of primary palmar hyperhidrosis in adolescents. Chin Med J (Engl) 2007;120:2215-7. [PubMed]
- Ambrogi V, Campione E, Mineo D, et al. Bilateral thoracoscopic T2 to T3 sympathectomy versus botulinum injection in palmar hyperhidrosis. Ann Thorac Surg 2009;88:238-45. [PubMed]
- Chen YB, Ye W, Yang WT, et al. Uniportal versus biportal video-assisted thoracoscopic sympathectomy for palmar hyperhidrosis. Chin Med J (Engl) 2009;122:1525-8. [PubMed]
- Georghiou GP, Berman M, Bobovnikov V, et al. Minimally invasive thoracoscopic sympathectomy for palmar hyperhidrosis via a transaxillary single-port approach. Interact Cardiovasc Thorac Surg 2004;3:437-41. [PubMed]
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [PubMed]
- Ibrahim M, Menna C, Andreetti C, et al. Bilateral single-port sympathectomy: long-term results and quality of life. Biomed Res Int 2013;2013:348017.
- Schmidt J, Bechara FG, Altmeyer P, et al. Endoscopic thoracic sympathectomy for severe hyperhidrosis: impact of restrictive denervation on compensatory sweating. Ann Thorac Surg 2006;81:1048-55. [PubMed]
- Gossot D, Kabiri H, Caliandro R, et al. Early complications of thoracic endoscopic sympathectomy: a prospective study of 940 procedures. Ann Thorac Surg 2001;71:1116-9. [PubMed]
- Lesèche G, Castier Y, Thabut G, et al. Endoscopic transthoracic sympathectomy for upper limb hyperhidrosis: limited sympathectomy does not reduce postoperative compensatory sweating. J Vasc Surg 2003;37:124-8. [PubMed]
- Yoon DH, Ha Y, Park YG, et al. Thoracoscopic limited T-3 sympathicotomy for primary hyperhidrosis: prevention for compensatory hyperhidrosis. J Neurosurg 2003;99:39-43. [PubMed]
- Riet M, Smet AA, Kuiken H, et al. Prevention of compensatory hyperhidrosis after thoracoscopic sympathectomy for hyperhidrosis. Surg Endosc 2001;15:1159-62. [PubMed]
- Ibrahim M, Menna C, Andreetti C, et al. Two-stage unilateral versus one-stage bilateral single-port sympathectomy for palmar and axilllary hyperhidrosis. Interact Cardiovasc Thorac Surg 2013;16:834-8. [PubMed]
- Jeganathan R, Jordan S, Jones M, et al. Bilateral thoracoscopic sympathectomy: results and long-term follow-up. Interact Cardiovasc Thorac Surg 2008;7:67-70. [PubMed]
- Lin CC, Mo LR, Lee LS, et al. Thoracoscopic T2-sympathetic block by clipping--a better and reversible operation for treatment of hyperhidrosis palmaris: experience with 326 cases. Eur J Surg Suppl 1998.13-6. [PubMed]
- Cramer MN, Jay O. Compensatory hyperhidrosis following thoracic sympathectomy: a biophysical rationale. Am J Physiol Regul Integr Comp Physiol 2012;302:R352-6. [PubMed]