Minimally invasive mediastinal surgery
Introduction
In the past, mediastinal surgery was associated with the necessity of a maximum exposure, which was accomplished with various approaches, including median sternotomy, posterolateral thoracotomy, unilateral anterior thoracotomy with partial sternotomy (hemi-clamshell), and bilateral anterior thoracotomy with transverse sternotomy (clamshell). These approaches were associated with high morbidity and long post-operative stays. In the early 1990s, many surgical fields, such as thoracic surgery, observed the development of minimally invasive techniques.
Video-assisted thoracic surgery (VATS) is a minimally invasive technique that permits reduced operative trauma compared to open surgery, but is not routinely performed as a therapeutic approach for mediastinal mass resections. Some surgeons are hesitant to utilize VATS, especially for anterior and upper mediastinal lesions, as the mediastinum is an impervious space in the thorax that contains vital structures at risk of injury during surgery (1-4). Furthermore, although VATS gives clear benefits, it also has some disadvantages for the operator, such as long instruments placed through fixed entry points creating a fulcrum effect, bi-dimensional screen vision of the surgical field, and the creation of an unnatural visual effect that can cause the surgeon to lose orientation and the eye-hand-target axis when the camera is under an assistant’s control.
In order to overcome these limitations, some robotic systems have been developed over the last two decades. For the purposes of this article, we define robotic surgery as a surgical procedure that utilizes a device with computer enhanced technology, during which the interaction between surgeon and patient is under the direct control of the surgeon. The Automated Endoscopic System for Optimal Positioning was the first robotic arm approved by the US Food and Drug Administration (FDA) to be used in laparoscopic surgery (5). Subsequently, the same company (Computer Motion Inc., Goleta, CA, USA) developed the ZEUS system to assist surgeons in minimally invasive surgery (6,7). At the same time, the da Vinci Surgical System was developed by Intuitive Surgical (Sunnyvale, CA, USA) and cleared by the FDA for laparoscopy, thoracoscopy and intracardiac mitral valve repair. Currently, the da Vinci Robotic system is the only complete surgical system applied in a wide range of surgical procedures.
The first thoracoscopic resection of mediastinal lesions, including thymectomy and a neurogenic tumor of the posterior sulci was reported by Roviaro et al. in 1994 (1). Eight years later, Yoshino et al. reported the first application of robotic system for the removal of the thymus and a posterior mediastinal mass (8,9). Since then, the application of minimally invasive approaches, including robotic systems, has been increasingly spreading worldwide. Herewith, we describe the state of the art regarding different procedures, including thymectomy and posterior mediastinal lesion removal.
Thymectomy
The most frequent indications for thymectomy are myasthenia gravis (MG) and thymomatous disease, and the most common approach remained median sternotomy until the 1960s, when a series of almost 60 transcervical thymectomies was performed without significant complications or mortality (10). However, considering the results of some studies showing that thymic tissue could reside within the mediastinum or neck outside of the gland, many surgeons felt that maximum exposure techniques were more appropriate to ensure complete resection (11).
The debate between trans-cervical and trans-sternal thymectomies continued until a less invasive approach involving video-thoracoscopic surgery was developed in thoracic surgery (12,13). A large amount of data has been published regarding minimally invasive thymectomy for MG, reporting interesting results. Both unilateral and bilateral VATS techniques have been applied for thymectomy, and have been demonstrated to result in less operative trauma, lower morbidity and shorter hospital stays when compared to the trans-sternal approach (14-19). The operative times were acceptable and conversion rate was low (Table 1). Additionally, Rückert et al. reported better preservation of lung function with minimally invasive approaches compared to median sternotomy (20). Some authors preferred the right-side approach due to the relatively larger chest cavity and optimal visualization of the superior vena cava and the right phrenic nerve (21,22). Other authors preferred the left-side approach, underlining that the left pericardio-phrenic and aorto-pulmonary window fat tissues can be more easily reached from the left (23,24).
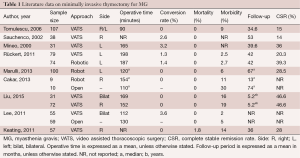
Full table
It is important to say that one of the most important keypoints of the minimally invasive technique for the treatment of MG is represented by the neurological outcomes, which are related to the necessity of resecting all thymus and peri-thymic fat tissue [extended thymectomy according to the Masaoka criteria (25), considering the distribution of thymic tissue and ectopic thymic tissue (11)].
Lee et al. compared the trans-sternal approach with bilateral VATS, observing no differences in the extent of the resected specimen (19). Meanwhile, Liu et al. reported that unilateral VATS offered long-term neurological outcomes equivalent to that of bilateral VATS (21). Other authors reported interesting mid-term neurologic results on 107 consecutive non-thymomatous myasthenic patients who underwent unilateral VATS, with the sidedness of the approach based on individual cases and the computed tomography (CT) status of the thymus (26). However, VATS has some disadvantages, including planar vision of the operative field and the lack of ability to articulate the tips of the thoracoscopic instruments. Robotic systems can overcome these limitations, combining the advantages of VATS (less operative trauma, short hospital stay and cosmetic results) with three-dimensional (3D) vision and the ability to articulate the tips of the instrument inside the chest cavity. Excellent results were reported in several series (23,27-30), showing low conversion rate, low morbidity and adequate operative time. In a recent study, Cakar et al. observed that robotic thymectomy in patients with MG provided at least the same benefits as open trans-sternal thymectomy with regard to improvement of neuro-muscular symptoms and drug dose reduction, but with a lower rate of complication and reintervention: the clinical outcomes of remission favored the robotic approach (31). Thus, the authors suggested that, with a unilateral robotic technique, it is possible to dissect and remove at least the same amount of mediastinal fat tissue, in addition to thymic tissue, as with the trans-sternal approach. Additionally, Rückert et al. reported an improved neurological outcome for the robotic thymectomy in respect to thoracoscopic thymectomy, for patients affected by MG (32). Another criticism is represented by thymomatous disease. Since 1993, some papers have attested to the possibility of performing thymectomy for Masaoka Stage I and II thymomas with VATS (1-3). The first paper describing the robotic resection of a Masaoka Stage I thymoma was published by Yoshino et al. in 2001. In spite of this data, few papers evaluated the role of minimally-invasive approach and some authors advocated the necessity of median sternotomy due to the high risk of tumor spread inside the chest cavity (8). In 2010, Agasthian et al. reported a local recurrence rate of 3.4% in patients who underwent VATS thymectomy for thymoma (33), while two recent papers compared the VATS and the open approaches for thymomas (16,34). Cheng et al. observed no local or pleural disease recurrence for Stage II thymoma, either in the open or in the VATS groups (16), and similar outcomes were published by Pennathur et al. (34) in a larger comparative study, reporting no significant difference in disease recurrence and overall survival between the two groups. Comparable results were obtained by He et al. and Liu et al. in comparative studies, which analyzed the trans-sternal approach and the VATS one (35,36). In another study in 2012, focused on robotic thymectomy for thymoma, the authors emphasized the excellent visualization provided of the mediastinal field, including all thymic and peri-thymic tissues and the thymoma capsule, that allowed a safe manipulation of mediastinal tissue. In these series, no disease recurrence was observed (37). A recent paper by Ye et al. compared 25 VATS thymectomies and 21 robotic thymectomies for stage I thymoma. Robotic thymectomy appeared to be feasible, safe and equally minimally invasive as VATS, and resulted in a shorter drainage period and reduced hospital stay compared with the VATS approach (38). A multicentre European study on 79 thymomas resected with the robotic approach showed good surgical results with no mortality, low morbidity, and short hospital need to insert reference for this study. With regard on oncologic results, only one case of disease relapse occurred (1.3%), a percentage that is comparable with other thoracoscopic series (29) (Table 2). However, considering the indolent nature of thymomatous disease, long follow-up period is necessary in order to assess the long-term oncologic results. Thus, further studies on larger series are necessary to confirm the good results of minimally-invasive approaches.
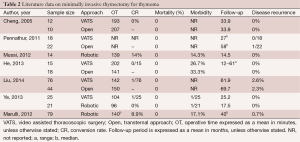
Full table
Posterior mediastinal lesion resection
The most frequent lesions occurring in the posterior mediastinum are represented by neurogenic tumors, which are generally asymptomatic and diagnosed incidentally. These tumors are usually benign and arise in the costovertebral sulci from sensitive fibres of intercostal nerves, from the sympathetic chain or, less frequently, from the vagus nerve. In the past, thoracotomy was the traditional surgical approach, but at the beginning of the 1990s, VATS was introduced to the thoracic field and applied for the removal of posterior mediastinal lesions (1). VATS minimized the trauma incision, reduced hospital stays, and provided an appropriate view of the posterior mediastinum (39-41). However, the removal of dumbbell-type lesions may be considered challenging (42) and, in this sense, a combined neurosurgical and thoracoscopic approach would be an excellent strategy (43).
Another criticism is represented by tumors lodged in the superior sulcus, close to the stellate ganglion, where careful bipolar dissection is recommended. In this setting, the features of the robotic system could help surgeons. At the beginning of the 21st century, some papers reported the removal of a posterior-mediastinal mass with the aid of robotic systems, emphasizing its safety and feasibility (9,44). Subsequently, studies on the application of robotic systems for removal of mediastinal lesion, including neurogenic tumors, have highlighted features such as depth perception, tremor filtration and 7-degree articulation of the instrument tips that have permitted a precise isolation of the anatomic structures and safe manipulation of the tissues. Melfi et al. reported no mortality and a morbidity rate of 7.2% (30). A heterogeneous study of Cerfolio et al., comprising different kind of surgical procedures, reported no mortality and low morbidity rate (12%): one esophageal perforation, four atrial fibrillations, two pneumothoraces, one gout and one prolonged air leak (45). Nakamura et al. published their Japanese experience with robotic-assisted resection of posterior mediastinal masses, where no conversions or complications were reported (46).
Other small studies have demonstrated that operating tumors in the posterior mediastinum is feasible with the robotic-assisted approach (47,48). Other pathologies uncommon to the posterior mediastinum, such as thyroid goiters, have also been resected using robotic-assisted systems (49,50). Additionally, in 2010, Chon et al. observed that robotic-assisted resection of double primary tumors within different areas of the mediastinum (anterior and posterior) is possible with a single-stage operation (51).
Ectopic mediastinal parathyroid adenoma removal
Primary hyperparathyroidism is the most common cause of hypercalcemia. Usually, the culprit gland or glands are located in the cervical region, but can also be found in ectopic locations in 15% to 20% of cases (52), including the mediastinum, which in the past was accessed through a median sternotomy or thoracotomy.
With improved technology, more minimally invasive approaches such as VATS are being used (53,54). Additionally, several case series have reported the application of robotic systems to resect ectopic parathyroid glands located in the mediastinum, demonstrating its safety and feasibility (55-58). Profanter et al. underlined the advantage of the robotic-assisted system in respect to VATS when dissecting tissue in areas such as the aortopulmonary window (55). Transient weakness of the left recurrent laryngeal nerve was the only postoperative complication recorded in these series, and this complication had completely resolved by 8 months (56).
Other mediastinal pathology
At the beginning of the last century, VATS began to be applied also for resection of lesions occurring less commonly within the mediastinum, such as pleuropericardial cysts, lipomas, teratomas or fibrous tumors of the mediastinum. Roviaro et al. reported the application of VATS for a wide range of mediastinal lesions, highlighting that VATS provides good exposure of the entire mediastinum, ensures adequate room for surgical manoeuvers, and results in greatly reduced surgical trauma (1). Martinod et al. reported a series of bronchogenic cysts removed with thoracoscopic approach (59), while Umemori et al. reported the successful VATS resection of pleuropericardial cysts of the mediastinum (60).
In 2004, Bodner et al. reported the application of the robotic system for the treatment of various mediastinal lesions, and Meehan et al. described their experience with robotic-assisted resections of mediastinal lesions in children, including teratomas and germ cell tumors (61). Another paper by Melfi et al. documented the resection of nine pleuropericardial cysts and three teratomas (30). The results from both of these series demonstrate that even uncommon pathologies within the mediastinal borders can be extirpated with good results.
Conclusions
During the 20th century, less invasive surgical approaches were developed to access the mediastinum. VATS has the potential to reduce morbidity, postoperative pain levels and length of hospital stay. However, this technique has some limitations. These include the planar vision intraoperatively, with the loss of depth perception; the limited maneuverability of thoracoscopic instruments, making it difficult to operate around corners; the fulcrum effect of instruments inserted through the chest wall; and the non-ergonomic positions that the surgeon often has to adopt.
Robotic systems can be considered a natural progression of VATS, offering a high-definition 3D vision, tremor filtration and a 7-degree articulation of the instruments inside the chest cavity. These features appear to be highly useful in a remote and tiny space such as the mediastinum (Video 1).

Despite these positive results, randomized controlled studies are necessary in order to confirm the benefits of minimally invasive approaches, especially for thymectomy, both from a neurological and oncological point of view.
Acknowledgements
To Teresa Hung Key for linguistic accuracy checking.
Footnote
Conflicts of Interest: Franca Melfi is an official proctor for Intuitive Surgical. The other authors have no conflicts of interest to declare.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videothoracoscopic excision of mediastinal masses: indications and technique. Ann Thorac Surg 1994;58:1679-83; discussion 1683-4.
- Yim AP. Video-assisted thoracoscopic resection of anterior mediastinal masses. Int Surg 1996;81:350-3. [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [PubMed]
- Hazelrigg SR, Landreneau RJ, Mack MJ, et al. Thoracoscopic resection of mediastinal cysts. Ann Thorac Surg 1993;56:659-60. [PubMed]
- Omote K, Feussner H, Ungeheuer A, et al. Self-guided robotic camera control for laparoscopic surgery compared with human camera control. Am J Surg 1999;177:321-4. [PubMed]
- Vassiliades TA Jr, Nielsen JL. Alternative approaches in off-pump redo coronary artery bypass grafting. Heart Surg Forum 2000;3:203-6. [PubMed]
- Stephenson ER Jr, Sankholkar S, Ducko CT, et al. Robotically assisted microsurgery for endoscopic coronary artery bypass grafting. Ann Thorac Surg 1998;66:1064-7. [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Video-assisted thoracoscopic extirpation of a posterior mediastinal mass using the da Vinci computer enhanced surgical system. Ann Thorac Surg 2002;74:1235-7. [PubMed]
- Banowsky LH, Braun WE, Crile G, et al. Transcervical thymectomy in renal transplant recipients: surgical complications and possible effect on long-term allograft survival. Trans Am Assoc Genitourin Surg 1975;67:103-5. [PubMed]
- Fukai I, Funato Y, Mizuno T, et al. Distribution of thymic tissue in the mediastinal adipose tissue. J Thorac Cardiovasc Surg 1991;101:1099-102. [PubMed]
- Mack MJ, Landreneau RJ, Yim AP, et al. Results of video-assisted thymectomy in patients with myasthenia gravis. J Thorac Cardiovasc Surg 1996;112:1352-9; discussion 1359-60. [PubMed]
- Roviaro G, Varoli F, Nucca O, et al. Videothoracoscopic approach to primary mediastinal pathology. Chest 2000;117:1179-83. [PubMed]
- Savcenko M, Wendt GK, Prince SL, et al. Video-assisted thymectomy for myasthenia gravis: an update of a single institution experience. Eur J Cardiothorac Surg 2002;22:978-83. [PubMed]
- Manoly I, Whistance RN, Sreekumar R, et al. Early and mid-term outcomes of trans-sternal and video-assisted thoracoscopic surgery for thymoma. Eur J Cardiothorac Surg 2014;45:e187-93. [PubMed]
- Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest 2005;128:3010-2. [PubMed]
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic thymectomy in autoimmune myasthesia: results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [PubMed]
- Lee CY, Kim DJ, Lee JG, et al. Bilateral video-assisted thoracoscopic thymectomy has a surgical extent similar to that of transsternal extended thymectomy with more favorable early surgical outcomes for myasthenia gravis patients. Surg Endosc 2011;25:849-54. [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [PubMed]
- Liu Z, Yang J, Lin L, et al. Unilateral video-assisted thoracoscopic extended thymectomy offers long-term outcomes equivalent to that of the bilateral approach in the treatment of non-thymomatous myasthenia gravis. Interact Cardiovasc Thorac Surg 2015;21:610-5. [PubMed]
- Keating CP, Kong YX, Tay V, et al. VATS thymectomy for nonthymomatous myasthenia gravis: standardized outcome assessment using the myasthenia gravis foundation of America clinical classification. Innovations (Phila) 2011;6:104-9. [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Experience with the “da Vinci” robotic system for thymectomy in patients with myasthenia gravis: report of 33 cases. Ann Thorac Surg 2006;81:455-9. [PubMed]
- Li Y, Wang J. Left-sided approach video-assisted thymectomy for the treatment of thymic diseases. World J Surg Oncol 2014;12:398. [PubMed]
- Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg 1996;62:853-9. [PubMed]
- Tomulescu V, Ion V, Kosa A, et al. Thoracoscopic thymectomy mid-term results. Ann Thorac Surg 2006;82:1003-7. [PubMed]
- Fleck T, Fleck M, Müller M, et al. Extended videoscopic robotic thymectomy with the da Vinci telemanipulator for the treatment of myasthenia gravis: the Vienna experience. Interact Cardiovasc Thorac Surg 2009;9:784-7. [PubMed]
- Augustin F, Schmid T, Bodner J. The robotic approach for mediastinal lesions. Int J Med Robot 2006;2:262-70. [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [PubMed]
- Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501-4; discussion 504-5. [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [PubMed]
- Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann 2010;18:234-9. [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [PubMed]
- He Z, Zhu Q, Wen W, et al. Surgical approaches for stage I and II thymoma-associated myasthenia gravis: feasibility of complete video-assisted thoracoscopic surgery (VATS) thymectomy in comparison with trans-sternal resection. J Biomed Res 2013;27:62-70. [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6; discussion e47.
- Ye B, Tantai JC, Li W, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol 2013;11:157. [PubMed]
- Abdel Rahman AR, Sedera MA, Mourad IA, et al. Posterior mediastinal tumors: outcome of surgery. J Egypt Natl Canc Inst 2005;17:1-8. [PubMed]
- Kumar A, Kumar S, Aggarwal S, et al. Thoracoscopy: the preferred approach for the resection of selected posterior mediastinal tumors. J Laparoendosc Adv Surg Tech A 2002;12:345-53. [PubMed]
- Yamaguchi M, Yoshino I, Fukuyama S, et al. Surgical treatment of neurogenic tumors of the chest. Ann Thorac Cardiovasc Surg 2004;10:148-51. [PubMed]
- Negri G, Puglisi A, Gerevini S, et al. Thoracoscopic techniques in the management of benign mediastinal dumbbell tumors. Surg Endosc 2001;15:897. [PubMed]
- Ciriaco P, Negri G, Bandiera A, et al. Videothoracoscopic resection of benign neurogenic tumors of the posterior mediastinum. Innovations (Phila) 2006;1:332-4. [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [PubMed]
- Nakamura H, Suda T, Ikeda N, et al. Initial results of robot-assisted thoracoscopic surgery in Japan. Gen Thorac Cardiovasc Surg 2014;62:720-5. [PubMed]
- Kajiwara N, Kakihana M, Usuda J, et al. Extended indications for robotic surgery for posterior mediastinal tumors. Asian Cardiovasc Thorac Ann 2012;20:308-13. [PubMed]
- Rea F, Schiavon M, Di Chiara F, et al. Single-institution experience on robot-assisted thoracoscopic operations for mediastinal diseases. Innovations (Phila) 2011;6:316-22. [PubMed]
- Al-Mufarrej F, Margolis M, Tempesta B, et al. Novel thoracoscopic approach to posterior mediastinal goiters: report of two cases. J Cardiothorac Surg 2008;3:55. [PubMed]
- Podgaetz E, Gharagozloo F, Najam F, et al. A novel robot-assisted technique for excision of a posterior mediastinal thyroid goiter: a combined cervico-mediastinal approach. Innovations (Phila) 2009;4:225-8. [PubMed]
- Chon SH, Shinn SH, Song DS, et al. Double primary tumor, thymic mass and posterior mediastinal neurogenic tumor, in a patient with acute pancreatitis performed with single-staged robotic-assisted thoracoscopic surgery. Surg Laparosc Endosc Percutan Tech 2010;20:e176-8. [PubMed]
- Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 2006;191:418-23. [PubMed]
- Kim YS, Kim J, Shin S. Thoracoscopic removal of ectopic mediastinal parathyroid adenoma. Korean J Thorac Cardiovasc Surg 2014;47:317-9. [PubMed]
- Adachi Y, Nakamura H, Taniguchi Y, et al. Thoracoscopic resection with intraoperative use of methylene blue to localize mediastinal parathyroid adenomas. Gen Thorac Cardiovasc Surg 2012;60:168-70. [PubMed]
- Profanter C, Schmid T, Prommegger R, et al. Robot-assisted mediastinal parathyroidectomy. Surg Endosc 2004;18:868-70. [PubMed]
- Bodner J, Profanter C, Prommegger R, et al. Mediastinal parathyroidectomy with the da Vinci robot: presentation of a new technique. J Thorac Cardiovasc Surg 2004;127:1831-2. [PubMed]
- Ismail M, Maza S, Swierzy M, et al. Resection of ectopic mediastinal parathyroid glands with the da Vinci robotic system. Br J Surg 2010;97:337-43. [PubMed]
- Timmerman GL, Allard B, Lovrien F, et al. Hyperparathyroidism: robotic-assisted thoracoscopic resection of a supernumary anterior mediastinal parathyroid tumor. J Laparoendosc Adv Surg Tech A 2008;18:76-9. [PubMed]
- Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg 2000;69:1525-8. [PubMed]
- Umemori Y, Kotani K, Makihara S. Video-assisted thoracoscopical surgery for pericardial cyst: report of two cases. Kyobu Geka 2001;54:1125-7. [PubMed]
- Meehan JJ, Sandler AD. Robotic resection of mediastinal masses in children. J Laparoendosc Adv Surg Tech A 2008;18:114-9. [PubMed]





