Biomarkers and prognostic factors for mesothelioma
Malignant pleural mesothelioma (MPM) desperately needs non-invasive, accurate prognostication for many reasons: a median survival of 12 months with treatment with first line therapy (1); a median survival of 24 months at best when treated in a multimodal approach with either neoadjuvant chemotherapy and surgery with or without radiation therapy or postoperative chemotherapy (2); a staging system that is not ideal, considering the diffuse nature of the disease and its variable biology (3); difficult, non-R0 surgical cytoreductions that, even with specialized centers have times to progression ranging from 7-12 months, and operative mortalities of 5% (4). MPM patients tend to be older individuals who are frequently functionally impaired and may have difficulty with aggressive therapy; however, there are a cadre of MPM patients who, with favorable biology and a multimodal approach, benefit from intense therapy. Prognostication in MPM must be able to differentiate among patients, hopefully at the time of diagnosis, in whom it is justified to offer potentially hazardous standards of care or novel protocols. If such prognostication implies a short time to death, either palliative therapy or no therapy may be appropriate; however, if prognostic factors indicate that long term survival is possible, a more aggressive approach may be prescribed. Obviously, however, prognostication cannot work in a vacuum and with time prognostication must be closely linked with prediction of response to therapy, in that a patient with a poor prognostic, but predictably sensitive to therapy, tumor may actually benefit from such therapy.
Prognostication in MPM has been approached by studying many variables, usually one at a time, at many centers, all with limited numbers of patients. Univariate and multivariate analyses are performed, yet the majority of the findings remain unvalidated in other MPM populations. The variables can be purely clinical, such as patient demographics, which are frequently combined with standard laboratory values including white blood cell count or platelet count. Other investigators have concentrated on radiologic parameters at presentation as determined by scrutiny of computerized tomograms (CT) or positron emission tomography (PET) alone or fused with CT. Finally, a molecular pathologic approach, using state of the art platforms such as genomics, microRNA, epigenetics, or proteomics is used in order to define single or combinations of candidate prognostic biomarkers from tissue or blood.
Clinical factors for the prognostication of MPM
The best-known clinical prognostic scoring systems for MPM have originated from European Organisation for Research and Treatment of Cancer (EORTC) and Cancer and Leukemia Group B (CALGB) (5), and use a combination of biological and clinical factors (Table 1). Poor performance status, non-epithelioid histology, male gender, low hemoglobin, high platelet count, high white blood cell count, and high lactate dehydrogenase (LDH) were found to be poor prognostic indicators in mesothelioma. The EORTC model was validated at St. Bartholomew’s Hospital in a group of 145 patients treated in sequential phase II chemotherapy trials (16). As seen in Table 1, there have been a number of mostly retrospective analyses of clinical variables alone or in combination with clinical variables laboratory parameters since the EORTC and CALGB studies were reported. A recurring theme in patients who have not had surgical resection includes non-epithelial histotype, low hemoglobin, and high WBC as poor prognostic indicators in these studies. As a follow-up on the EORTC data, a prognostic index for progression free survival revealed that age, histotype, stage, performance status, hemoglobin (9) and WBC levels were independent predictors time to progression. For MPM patients undergoing surgical resection, an IASLC/International Mesothelioma Interest Group sponsored retrospective registry of 3,101 patients from 15 centers on 4 continents has described “core” prognostic variables as stage, histotype, gender, age, and treatment intent (curative or palliative) (Rusch in press). The prognostic significance of other demographic factors including adjuvant therapy, WBC, hgb, smoking history, asbestos history, performance status, chest pain, and weight loss are also being investigated.
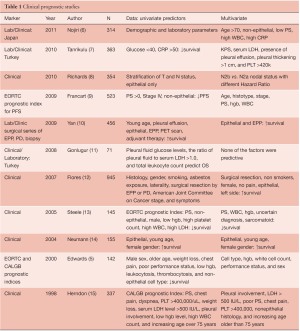
Full table
Radiographic and nuclear imaging prognostic studies in mesothelioma
Quantification of the standardized uptake value (SUV) for PET scanning as well as novel CT techniques have also been investigated in mesothelioma for prognostic reliability (Table 2). Low SUV and epithelial histology predict the best survival, whereas high SUV and nonepithelial histology indicate the worst survival. In a multivariate analysis of 65 patients with MPM, median survival was 14 and 24 months for the high and low SUV groups, respectively. High SUV tumors were associated with 3.3 times greater risk of death than low SUV tumors (P=0.03) (21). Gerbaudo et al. (22) reported that the intensity of FDG uptake by mesothelioma correlates poorly with histology, but well with surgical stage. A recent “best evidence” report from Sharif et al.(18) addressed whether PET is useful in the diagnosis and prognosis of MPM. Altogether only 15 of 136 papers represented the best evidence studies, and these revealed that malignant disease had a higher SUV (6.5±3.4 vs. 0.8±0.6; P<0.001) than benign pleural disease. Shorter median survival (9.7 vs. 21 months; P=0.02) was associated with high SUV (>10) compared to low SUV (<10). Overall, PET accurately diagnoses MPM and predicts survival and disease recurrence. With both PET as well as computerized tomographic studies, there has been an increasing emphasis on the role of volume measurements as a prognostic variable. In a study by Nowak et al. (20), volumetric FDG-PET parameters were more predictive of survival than tumor-node-metastasis staging in patients with non-sarcomatoid disease, suggesting that tumor volume and glycolytic activity may be more important determinants of prognosis in malignant pleural mesothelioma than anatomic extent of disease. Sarcomatoid histology, however, remained the strongest prognostic factor. In a study by Lee et al. (19), multivariate analysis adjusted for treatment modality showed that metabolic tumor volume and total lesion glycolysis were independent factors associated with tumor progression. Time to tumor progression was shorter in patients with a high volume-based parameter of PET than in those with a low value. Following up on earlier studies by Pass et al. (23) regarding the influence of tumor volume of survival and progression of surgically treated mesothelioma patients, Gill et al. (17) reported that CT-derived tumor volume can be used to stratify survival of 88 patients with epithelial mesothelioma after extrapleural pneumonectomy. In univariate analysis, tumor volume, hemoglobin concentration, platelet count, pathologic TNM category, and administration of adjuvant chemotherapy or radiation therapy met the criteria for inclusion in the reverse stepwise regression analysis. In the final model, tumor volume, hemoglobin concentration, and administration of adjuvant chemotherapy or radiotherapy were identified as independently associated with overall survival. Further multicenter validation of these CT volume findings is planned.
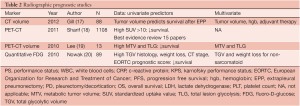
Full table
Molecular/pathologic prognostication
There are a multitude of studies investigating single or multiple genes in tissue, proteins, or circuiting blood based biomarkers for predicting MPM survival (Table 3). Global molecular prognostication of mesothelioma using gene expression array technology was first performed by Gordon et al. (55) using the 12,000 U95 Affymetrix gene chip. A four-gene expression ratio test was able to predict treatment-related patient outcome in mesothelioma, independent of the histologic subtype of the tumor. In a follow-up publication, these MPM prognostic genes and gene ratio-based prognostic tests predicted clinical outcome in a separate cohort of 39 independent MPM tumor specimens in a statistically significant manner (40). Using similar technology, Pass et al. (56) have reported a 27-gene expression array for mesothelioma prognostication. The groups predicted by the gene classifier recapitulated the actual time to progression and survival of the test set with 95.2% accuracy using tenfold cross validation. There has, however, been variability in the gene sets and results of these prognostic tests when used in other MPM cohorts. Affymetrix U133A microarray analysis on 99 pleural mesotheliomas from the Memorial Sloan-Kettering (MSK) Cancer Center revealed that advanced-stage, sarcomatous histology and P16/CDKN2A homozygous deletion to be significant, independent, adverse prognostic factors. Examination of the gene expression correlates of survival showed that more aggressive mesotheliomas expressed higher levels of Aurora kinases A and B. Moreover, evaluation of three recently published microarray-based outcome prediction models in the MSK cohort revealed accuracies from 63% to 67%, consistently lower than reported (47). At present, there are no validated gene sets for prognostication of MPM. Tumor tissue examination has revealed the presence of a single microRNA, mir-29c*, to be associated with improved time to progression and overall survival, but needs further validation (38). High nuclear grade has also been associated with poor survival in multivariate analyses (33). Some of the more recently published blood-based biomarkers have included neutrophil lymphocyte ratios (25,31,32) demonstrating poor prognosis for those patients having a low ratio, as well as elevated levels of SMRP and osteopontin showing correlation with shorter survival (46). Circulating endothelial cell (CEC) count has been positively correlated with intratumoral microvessel density and an elevated CEC count was significantly associated with a poor prognosis. Moreover, a multivariate analysis showed that higher CEC count was a significant and independent factor to predict a poor prognosis (24).
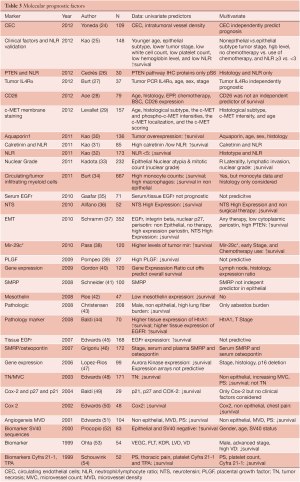
Full table
The future of prognostic biomarkers in MPM will most likely involve a multi-institutional consortium of centers which will harvest tissue, blood and other specimens in a protocol using the same standard operating procedures in order to minimize extraneous differences which could lead to false positive results. As new platforms develop, including analysis of other short RNA species, autoantibodies, and circulating tumor cells, along with new therapies, it will be crucial to make sure that an ongoing registry which incorporates robust demographics as well as documentation of specimen archiving be available to the Mesothelioma community. At this time the National Mesothelioma Virtual Tissue Bank (57) fulfills that role in the United States, and is adding new sites to ensure that reagents and tissues for MPM prognostication will be available.
Acknowledgements
Disclosure: Research Funding from NCI/NIH, DOD, CDC, Covidien, Mensanna, Rosetta Genomics, SomaLogic, Celera, SourceMDx, Fujirebio, Pfizer, Response Genetics, Meso Scale Diagnostics, Integrated Diagnostics, Transgenomics, Belluck and Fox, Stephen Banner Lung Foundation, Simmons Mesothelioma Foundation, Levi Phillips Konigsberg.
Medical Advisory Boards for Rosetta Genomics, Prometheus, Champions, Pinpoint Genomics, Precision Therapeutics, and GSK.
Research collaborations with Foundation Medicine, Response Genetics, Cynvezio.
Patents for use of osteopontin for diagnosis of mesothelioma; pending for microRNA for diagnosis/prognosis of mesothelioma; pending for EFEMP1 and mesothelioma diagnosis/prognosis.
References
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
- van Meerbeeck JP, Scherpereel A, Surmont VF, et al. Malignant pleural mesothelioma: the standard of care and challenges for future management. Crit Rev Oncol Hematol 2011;78:92-111.
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8.
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.
- Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax 2000;55:731-5.
- Nojiri S, Gemba K, Aoe K, et al. Survival and prognostic factors in malignant pleural mesothelioma: a retrospective study of 314 patients in the west part of Japan. Jpn J Clin Oncol 2011;41:32-9.
- Tanrikulu AC, Abakay A, Kaplan MA, et al. A clinical, radiographic and laboratory evaluation of prognostic factors in 363 patients with malignant pleural mesothelioma. Respiration 2010;80:480-7.
- Richards WG, Godleski JJ, Yeap BY,et al. Proposed adjustments to pathologic staging of epithelial malignant pleural mesothelioma based on analysis of 354 cases. Cancer 2010;116:1510-7.
- Francart J, Vaes E, Henrard S, et al. A prognostic index for progression-free survival in malignant mesothelioma with application to the design of phase II trials: a combined analysis of 10 EORTC trials. Eur J Cancer 2009;45:2304-11.
- Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619-24.
- Gonlugur U, Gonlugur TE. Prognostic factors for 100 patients with malignant pleural mesothelioma. Arch Environ Occup Health 2010;65:65-9.
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65.
- Steele JP. Prognostic factors for mesothelioma. Hematol Oncol Clin North Am 2005;19:1041-52, vi.
- Neumann V, Rutten A, Scharmach M, et al. Factors influencing long-term survival in mesothelioma patients--results of the German mesothelioma register. Int Arch Occup Environ Health 2004;77:191-9.
- Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723-31.
- Steele JP, Rudd RM. Malignant mesothelioma: predictors of prognosis and clinical trials. Thorax 2000;55:725-6.
- Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT-derived tumor volume. AJR Am J Roentgenol 2012;198:359-63.
- Sharif S, Zahid I, Routledge T, et al. Does positron emission tomography offer prognostic information in malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2011;12:806-11.
- Lee HY, Hyun SH, Lee KS, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 2010;17:2787-94.
- Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 2010;16:2409-17.
- Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography predicts survival in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2006;132:763-8.
- Gerbaudo VH, Britz-Cunningham S, Sugarbaker DJ, et al. Metabolic significance of the pattern, intensity and kinetics of 18F-FDG uptake in malignant pleural mesothelioma. Thorax 2003;58:1077-82.
- Pass HI, Temeck BK, Kranda K, et al. Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1998;115:310-7.
- Yoneda K, Tanaka F, Kondo N, et al. Circulating Endothelial Cell (CEC) as a diagnostic and prognostic marker in Malignant Pleural Mesothelioma (MPM). Ann Surg Oncol 2012;19:4229-37.
- Kao SC, Vardy J, Chatfield M, et al. Validation of prognostic factors in Malignant Pleural Mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales Dust Diseases Board. Clin Lung Cancer 2012. [Epub ahead of print].
- Cedrés S, Montero MA, Martinez P, et al. Exploratory analysis of activation of PTEN-PI3K pathway and downstream proteins in malignant pleural mesothelioma (MPM). Lung Cancer 2012;77:192-8.
- Burt BM, Bader A, Winter D, et al. Expression of interleukin-4 receptor alpha in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin Cancer Res 2012;18:1568-77.
- Aoe K, Amatya VJ, Fujimoto N, et al. CD26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin Cancer Res 2012;18:1447-56.
- Levallet G, Vaisse-Lesteven M, Le SN, et al. Plasma cell membrane localization of c-MET predicts longer survival in patients with malignant mesothelioma: a series of 157 cases from the MESOPATH Group. J Thorac Oncol 2012;7:599-606.
- Kao SC, Armstrong N, Condon B, et al. Aquaporin 1 is an independent prognostic factor in pleural malignant mesothelioma. Cancer 2012;118:2952-61.
- Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923-9.
- Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010;16:5805-13.
- Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260-71.
- Burt BM, Rodig SJ, Tilleman TR, et al. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer 2011;117:5234-44.
- Gaafar R, Bahnassy A, Abdelsalam I, et al. Tissue and serum EGFR as prognostic factors in malignant pleural mesothelioma. Lung Cancer 2010;70:43-50.
- Alifano M, Loi M, Camilleri-Broet S, et al. Neurotensin expression and outcome of malignant pleural mesothelioma. Biochimie 2010;92:164-70.
- Schramm A, Opitz I, Thies S, et al. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:566-72.
- Pass HI, Goparaju C, Ivanov S, et al. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res 2010;70:1916-24.
- Pompeo E, Albonici L, Doldo E, et al. Placenta growth factor expression has prognostic value in malignant pleural mesothelioma. Ann Thorac Surg 2009;88:426-31.
- Gordon GJ, Dong L, Yeap BY, et al. Four-gene expression ratio test for survival in patients undergoing surgery for mesothelioma. J Natl Cancer Inst 2009;101:678-86.
- Schneider J, Hoffmann H, Dienemann H, et al. Diagnostic and prognostic value of soluble mesothelin-related proteins in patients with malignant pleural mesothelioma in comparison with benign asbestosis and lung cancer. J Thorac Oncol 2008;3:1317-24.
- Roe OD, Creaney J, Lundgren S, et al. Mesothelin-related predictive and prognostic factors in malignant mesothelioma: a nested case-control study. Lung Cancer 2008;61:235-43.
- Christensen BC, Godleski JJ, Roelofs CR, et al. Asbestos burden predicts survival in pleural mesothelioma. Environ Health Perspect 2008;116:723-26.
- Baldi A, Mottolese M, Vincenzi B, et al. The serine protease HtrA1 is a novel prognostic factor for human mesothelioma. Pharmacogenomics 2008;9:1069-77.
- Edwards JG, Swinson DE, Jones JL, et al. EGFR expression: associations with outcome and clinicopathological variables in malignant pleural mesothelioma. Lung Cancer 2006;54:399-407.
- Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res 2007;13:2928-35.
- López-Ríos F, Chuai S, Flores R, et al. Global gene expression profiling of pleural mesotheliomas: overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res 2006;66:2970-9.
- Edwards JG, Swinson DE, Jones JL, et al. Tumor necrosis correlates with angiogenesis and is a predictor of poor prognosis in malignant mesothelioma. Chest 2003;124:1916-23.
- Baldi A, Santini D, Vasaturo F, et al. Prognostic significance of cyclooxygenase-2 (COX-2) and expression of cell cycle inhibitors p21 and p27 in human pleural malignant mesothelioma. Thorax 2004;59:428-33.
- Edwards JG, Faux SP, Plummer SM, et al. Cyclooxygenase-2 expression is a novel prognostic factor in malignant mesothelioma. Clin Cancer Res 2002;8:1857-62.
- Edwards JG, Cox G, Andi A, et al. Angiogenesis is an independent prognostic factor in malignant mesothelioma. Br J Cancer 2001;85:863-8.
- Procopio A, Strizzi L, Vianale G, et al. Simian virus-40 sequences are a negative prognostic cofactor in patients with malignant pleural mesothelioma. Genes Chromosomes Cancer 2000;29:173-9.
- Ohta Y, Shridhar V, Bright RK, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer 1999;81:54-61.
- Schouwink H, Korse CM, Bonfrer JM, et al. Prognostic value of the serum tumour markers Cyfra 21-1 and tissue polypeptide antigen in malignant mesothelioma. Lung Cancer 1999;25:25-32.
- Gordon GJ, Jensen RV, Hsiao LL, et al. Using gene expression ratios to predict outcome among patients with mesothelioma. J Natl Cancer Inst 2003;95:598-605.
- Pass HI, Liu Z, Wali A, et al. Gene expression profiles predict survival and progression of pleural mesothelioma. Clin Cancer Res 2004;10:849-59.
- Amin W, Parwani AV, Schmandt L, et al. National Mesothelioma Virtual Bank: a standard based biospecimen and clinical data resource to enhance translational research. BMC Cancer 2008;8:236.





