“Branch-first” continuous perfusion aortic arch replacement and its role in intra-operative cerebral protection
Surgery of the aortic arch remains one of the most challenging areas of cardiac surgery. Despite the advancements and refinements in surgical and perfusion techniques over the last 30 years, mortality, morbidity, and in particular the incidence of cerebral injury, remains higher than that reported in procedures performed on the more proximal aorta. The brain is the most oxygen-dependent organ in the body and many steps during aortic arch replacement have the potential to cause cerebral injury, either as a result of temporary interruption to its blood supply or the introduction of gaseous or particulate emboli. Traditional approaches have focused on the use of two concepts to limit cerebral injury during arch replacement. Firstly, the use of profound hypothermia to reduce cerebral metabolic demands combined with surgical haste to minimize periods of cerebral ischemia; and secondly, the addition of various techniques of cerebral perfusion (antegrade and/or retrograde) in an attempt to prolong the period of “safe” circulatory arrest.
Neuroprotection strategies
Deep hypothermic circulatory arrest (DHCA) undoubtedly provides the surgeon with an unrivalled bloodless and uncluttered operative field. However, this is associated with a number of clinically significant disadvantages. Firstly, the process of cooling and rewarming leads to significant prolongation of cardiopulmonary bypass times and associated postoperative organ dysfunction and coagulopathy (1). Secondly, during the rewarming period there is potential for cerebral ischemic reperfusion injury (2), impairment of normal cerebrovascular regulatory mechanisms (3-5), and the generation of excessive cerebral temperature gradients (6,7), all of which compound other sources of cerebral injury. Thirdly, the construction of open anastomoses during DHCA increases the risk of debris and air emboli being introduced into both the cerebral and distal circulation. Lastly, the time available to perform complex arch reconstruction during DHCA is limited since extended periods of arrest have been conclusively associated with proportionately increasing incidence of cerebral and end-organ injury (8). Of significance, even DHCA durations as short as 20 minutes have been associated with subtle higher cerebral dysfunction (9-11), potentially indicating that any period of DHCA can cause adverse neurological outcome. This time pressure may lead to either a technically imperfect or an inappropriately curtailed arch reconstruction, which in turn could result in early or late failures.
In an attempt to provide nutritive cerebral blood flow, and thus a prolongation of the “safe” DHCA period, many centers have adopted ancillary cerebral perfusion techniques. Whilst several high volume aortic centers have adopted the use of retrograde cerebral perfusion (RCP) coupled with DHCA successfully (12), there is both experimental and clinical evidence demonstrating the limited nutrient cerebral supply that RCP provides (13,14), and concerns have been raised over excessive retrograde perfusion pressures and associated cerebral edema compounding cerebral injury (15,16). In contrast, selective antegrade cerebral perfusion (SACP) is more widely used and does provide nutritive cerebral flow (17-19). The techniques fall into 2 main groups: (I) either selective cannulation of individual branch vessels, or (II) the use of arch branch cannulation sites, such as the axillary, innominate or carotid artery. Nonetheless, both techniques have pitfalls. Direct cannulation of the branches through the open arch has the potential for introduction of atheromatous and/or air emboli. Furthermore, as there are periods of circulatory arrest prior to and following the introduction of the perfusion cannula, the profound hypothermia “umbrella” is still required. Finally, the passage of cannulas across the operative field contributes to reduction of exposure and ease of suturing. On the other hand, arch branch cannulation peripheral to the operative field (such as axillary artery) avoids many of these shortcomings but introduces its own drawbacks. Firstly, the entire period of arch reconstruction is done with a single inflow and is reliant on collaterals to carry flow to the contralateral hemisphere. This introduces the potential for ipsilateral hyperperfusion and/or contralateral hypoperfusion, depending on whether the flow is excessive or too meagre. Also, the cannulation/decannulation process does not in itself contribute to the anatomical arch reconstruction, so imposes additional technical steps, with their own inherent morbidity.
Importantly, both antegrade and retrograde cerebral perfusion techniques fail to address other vital organ perfusion and rely entirely on deep hypothermia for the protection of organs such as the kidney, liver and spinal cord. It has sometimes been argued that SACP provides distal organ perfusion via upper to lower body collaterals (18,20). However, it is dubious whether such collateral flow provides real nutritive flow in the context of an open distal anastomosis, where most such flow inevitably finds its way to the path of least resistance - directly into the pump sucker. Importantly, sub-lethal vital organ ischemia may be easily missed, as its effects are not as blatant as a stroke. Nonetheless, its impact may be just as lethal in the guise of coagulopathy, GI bleeding, sepsis and multi-organ failure.
Another area of major concern is the duration of cardiac ischemia. Typically, the heart is the first to be excluded from direct perfusion and the last to get re-perfused. While deep hypothermia is beneficial to myocardial protection, meticulous cardioplegia is vital to avoid low output syndrome after such prolonged cross clamp periods. Realistically, it is difficult to maintain such close attention to cardioplegia when the surgeon’s concentration is focused on complex arch reconstruction.
‘Branch-first' technique
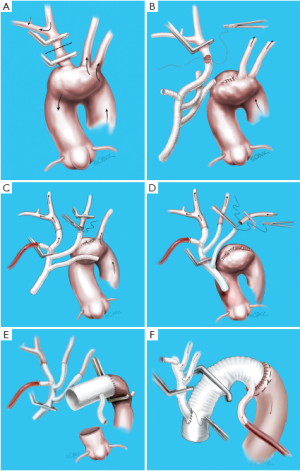
Whilst cerebral perfusion ancillaries have reduced the incidence of cerebral injury compared to DHCA alone, it is our view that these techniques represent a “reactive” approach to minimize the iatrogenic cerebral injury imposed by periods of global cerebral and body hypoperfusion during circulatory arrest. We believe that a more logical strategy to avoid cerebral and vital organ injury lies in a more “preventative” approach through the total avoidance of any period of circulatory arrest. Since 2005, our centre has adopted a “branch-first” continuous perfusion arch replacement technique. This approach has previously been described (21,22) and is presented in detail elsewhere in this issue. In essence the procedure consists of establishment of bypass using femoral inflow and moderate hypothermia (28 °C), serial disconnection and reconstruction of each arch branch (proceeding from innominate to left subclavian) using a trifurcation arch graft with a perfusion side arm port (TAPP graft, Vascutek Ltd., Renfrewshire, Scotland, UK, Figure 1), clamping of the proximal descending aorta and construction of the distal arch anastomosis, completion of aortic root reconstruction, and finally connection of the common stem of the trifurcation graft to the ascending aortic graft (Figure 2). Importantly there are no periods of global circulatory arrest. Individual arch branches are clamped for brief periods (typically around 10 minutes) before direct reperfusion is resumed from the side arm of the TAPP graft. Note that the other 2 branch vessels are perfused during this brief clamping of the branch undergoing reconstruction, and provide ample collateral supply to the latter’s territory (Figure 3). Note also that there is no interruption to cerebral perfusion during the anastomosis of the TAPP graft to the ascending graft, as the perfusion side arm is located distally. In addition, cardiac perfusion is maintained during the whole phase of arch branch reconstruction, significantly shortening cardiac ischemic time. Finally, distal organ perfusion is maintained throughout the whole operation. Ancillary benefits of the “branch-first” technique include the avoidance of deep hypothermia, with the potential to decrease coagulopathy and other complications of prolonged bypass. Additionally, there are advantages of individual arch branch over arch “island” reconstruction techniques (23), including easily accessible suture lines constructed in more normal arterial tissue.
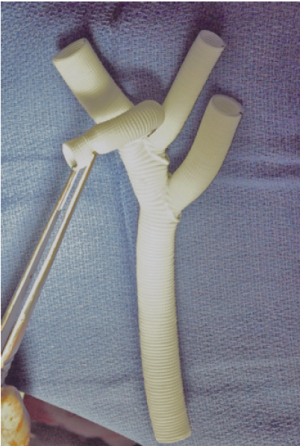

A basic premise of the “branch-first” technique is the richness of the collateral network between the 3 arch branches (Figure 3), thereby permitting a short period of exclusion of 1 branch while the other 2 branches service its territory. In contrast, 2 of 3 branches are excluded from perfusion for the whole period of arch reconstruction during unilateral SACP techniques. It is also vital to note that unlike the situation pertaining to carotid endarterectomy, the circle of Willis is only one of several pathways to cross the midline of the body. Notwithstanding this, even in carotid endarterectomy, hypoperfusion stroke is very uncommon with clamp periods less than 15 minutes (24-26). We also know that permanent common carotid ligation in neonatal extracorporeal membrane oxygenation (27,28) or innominate ligation for trachea-innominate fistula (29) carry surprisingly low stroke rates. Additional protection is built into the “branch-first” technique by the use of systemic heparinization, moderate cooling, full cardiopulmonary bypass with the ability to vary flow and pressure at will, and the availability of a multitude of cerebral monitoring technology including near-infrared spectroscopy. Finally, as anastomoses between the individual arch branches and the TAPP graft are entirely accessible after completion and pressurisation, each anastomosis can be done expeditiously (typically under 10 minutes) without fear of having subsequent inaccessible bleeding. The more recent development of a perfusion side arm in the TAPP graft (Figure 1) has completely removed the need for preliminary axillary cannulation for the provision of inflow to the trifurcation graft.
Maintenance of distal perfusion throughout arch reconstruction, and cardiac perfusion for a significant part of reconstruction, is an added benefit of the branch first technique. During the period of arch branch reconstruction, the arch itself is intact allowing maintained perfusion of both the heart and vital organs via the main arterial cannula. Completion of the arch branch reconstruction affords extra space by moving the arch branches out of the immediate operative field and increased mobility of the de-branched arch. Thus, it becomes relatively straightforward to clamp the proximal descending aorta, allowing maintenance of distal perfusion while the main distal arch anastomosis is completed. It is only at this stage that cardiac exclusion starts and cardioplegia become necessary.
Clinical outcomes
Our clinical results using the “branch-first” technique have been recently reported (21,22). In a 5-year period (2005-2010) we performed 42 “branch-first” arch reconstructions, including 44% with acute type A aortic dissection. There were 2 (4.8%) deaths. One was an 86-year-old female with a delayed presentation of acute type A dissection with right ventricular infarction. She died as a consequence of low cardiac output state despite grafting the right coronary artery. The other patient presented with advanced peripheral and bowel ischemia, also due to acute type A dissection. He died as a result of persistent gut infarction. There were no deaths in elective cases. Three patients (7.1%) experienced postoperative neurological events. Two of these were transient events comprised of amaurosis fugax in one patient and left hemiparesis in the other, both of which completely resolved. They were felt to be embolic events and cerebral imaging confirmed the absence of ischemic or watershed cerebral infarction. The third patient experienced short-term memory loss and expressive dysphasia, which did not completely resolve. This patient had known pre-existing cerebro-vascular disease and presented with a type A aortic dissection with fluctuating neurological signs. Thus, a permanent stroke incidence of 2.4% is acceptably low compared to reported rates in contemporary series, which range from 2.0% to 4.8% (9,30-33), and much lower than our previous reported experience with standard techniques (34).
Along with improved cerebral outcomes the “branch-first” technique has been associated with reduced myocardial and visceral injury, with 2% needing mechanical circulatory support and 7% requiring renal support. This is around one-third of the organ complication rate seen in our own historical experience with DHCA coupled with antegrade or retrograde cerebral perfusion (34). Coagulopathy has also been markedly reduced with 19% of cases not requiring any blood or blood products. Additionally, around half of the patients were extubated within the first 24 hours on return to ICU. This is further evidence not only for the excellent cardiac and other organ protection, but also the marked clarity with which the patients awoke, akin to routine cardiac surgery. Our intensive care colleagues have remarked on the dramatic reduction in postoperative delirium and acute brain syndromes with the adoption of the “branch-first” technique.
Advantages and disadvantages of the 'branch-first' technique
The “branch-first” technique removes the traditionally palpable time pressure of arch reconstruction and allows the operation to proceed in a logical, and complete fashion. The ability to systematically interrogate each branch anastomosis following its completion allows the surgeon to ensure optimal surgical technique and haemostasis at each step of the procedure. This is seen in our low incidence of blood product use in a complex cohort of patients including re-operations (21,22). Also, during our admittedly early follow-up no patient has required a re-operation for residual aortic pathology or intraoperative technical failures. We believe that the maintenance of cardiac and distal body perfusion in this technique allows even the most complex reconstructions to be completed meticulously in an unhurried fashion without concern over incomplete organ protection, thus providing complete correction of pathology and eliminating imperfections and compromises which may have otherwise been tolerated in view of time pressures.
There are a number of theoretical disadvantage of the “branch-first” technique. It may be argued that the use of femoral cannulation has the potential for retrograde dislodgement of atheromatous aortic debris that could be a potential source of embolic material leading to cerebral or other organ injury (35). Current outcomes and opinion on the practical risk of retrograde aortic perfusion is divided (35-40). However, the only situation with compelling evidence to suggest avoiding femoral cannulation is arch replacement for atherosclerotic rather than aneurysmal pathology (41). In fact it has been shown that ascending thoracic aneurysms and dissections are associated with decreased systemic atherosclerosis (42), a finding that supports the use of femoral cannulation in primary aneurysmal arch replacement. Our 2 embolic strokes occurred early in our experience and may have resulted from overzealous handling of the arch and its branches. Consequently, we now practice a “no-touch” exposure technique of mobilizing the surrounding tissues away from the arch and its branches. Certainly, the combination of pre- and intra-operative imaging, including trans-esophageal and epi-aortic echocardiography and CT scanning, help select cases at higher risk of atheroembolism. In those patients we substitute alternative cannulation sites such as the axillary or carotid artery or direct ascending aorta.
Access to the left subclavian artery may be difficult in a deep chest or in the presence of a large arch aneurysm. A number of maneuvers are useful to improve access. These include a short extension of the sternotomy incision along the anterior border of the left sternocleidomastoid, and delaying left subclavian reconstruction until after the clamp has been applied to the proximal descending aorta with decompression of the arch. There may also be concern over difficulties with clamping of the descending thoracic aorta. If after complete de-branching of the arch the descending thoracic aorta is not able to be clamped, alternatives for distal control include intra-luminal balloon occlusion or even exposing and clamping of the descending aorta in the posterior pericardium behind the heart. While there may be scenarios such as contained leaks or large infected false aneurysms of the arch, which may make this technique hazardous, we have been able to use it in many complex and re-operative cases as the arch branches can be mobilized and reconstructed prior to opening the sternum, therefore ensuring maintenance of cerebral perfusion even if aortic or cardiac injury is encountered on re-entry. The potential for kinks or twists of the limbs of the TAPP graft can be avoided by aggressively trimming the length of each limb after fully stretching it. In practice we have found that the majority of complex scenarios are suited to this technique.
We believe that the “branch-first” technique represents the next step in the evolution of aortic arch surgery. We have shown its safety and reported its excellent results both in elective and emergent situations. The technique simplifies aortic arch replacement while removing the need for deep hypothermia, circulatory arrest, extended periods of cardiopulmonary bypass and cerebral, cardiac and other organ circulatory exclusion. In addition to the avoidance of circulatory arrest and the associated additional time pressure on the surgeon, the minimization of additional steps involved in axillary arterial cannulation and the ability to interrogate each step of the arch reconstruction in a meticulous fashion allows the technique to be adopted easily and safely by the general cardiac surgeon without specialist aortic training. Our initial results are promising and hopefully with further experience and corroboration by other centers, the “branch-first” technique may prove to be superior to other traditional methods of arch replacement that rely on DHCA coupled with ancillary cerebral perfusion techniques.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Khaladj N, Peterss S, Pichlmaier M, et al. The impact of deep and moderate body temperatures on end-organ function during hypothermic circulatory arrest. Eur J Cardiothorac Surg 2011;40:1492-9; discussion 1499.
- Busto R, Dietrich WD, Globus MY, et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987;7:729-38.
- Greeley WJ, Ungerleider RM, Smith LR, et al. The effects of deep hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral blood flow in infants and children. J Thorac Cardiovasc Surg 1989;97:737-45.
- Neri E, Sassi C, Barabesi L, et al. Cerebral autoregulation after hypothermic circulatory arrest in operations on the aortic arch. Ann Thorac Surg 2004;77:72-9; discussion 79-80.
- Mault JR, Ohtake S, Klingensmith ME, et al. Cerebral metabolism and circulatory arrest: effects of duration and strategies for protection. Ann Thorac Surg 1993;55:57-63; discussion 63-4.
- Gordan ML, Kellermann K, Blobner M, et al. Fast rewarming after deep hypothermic circulatory arrest in rats impairs histologic outcome and increases NFkappaB expression in the brain. Perfusion 2010;25:349-54.
- Enomoto S, Hindman BJ, Dexter F, et al. Rapid rewarming causes an increase in the cerebral metabolic rate for oxygen that is temporarily unmatched by cerebral blood flow. A study during cardiopulmonary bypass in rabbits. Anesthesiology 1996;84:1392-400.
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion 28-31.
- Reich DL, Uysal S, Sliwinski M, et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg 1999;117:156-63.
- Ergin MA, Uysal S, Reich DL, et al. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887-90; discussion 1891-4.
- Krähenbühl ES, Immer FF, Stalder M, et al. Temporary neurological dysfunction after surgery of the thoracic aorta: a predictor of poor outcome and impaired quality of life. Eur J Cardiothorac Surg 2008;33:1025-9.
- Bavaria JE, Woo YJ, Hall RA, et al. Circulatory management with retrograde cerebral perfusion for acute type A aortic dissection. Circulation 1996;94:II173-6.
- Ehrlich MP, Hagl C, McCullough JN, et al. Retrograde cerebral perfusion provides negligible flow through brain capillaries in the pig. J Thorac Cardiovasc Surg 2001;122:331-8.
- Ye J, Yang L, Del Bigio MR, et al. Retrograde cerebral perfusion provides limited distribution of blood to the brain: a study in pigs. J Thorac Cardiovasc Surg 1997;114:660-5.
- Juvonen T, Zhang N, Wolfe D, et al. Retrograde cerebral perfusion enhances cerebral protection during prolonged hypothermic circulatory arrest: a study in a chronic porcine model. Ann Thorac Surg 1998;66:38-50.
- Reich DL, Uysal S, Ergin MA, et al. Retrograde cerebral perfusion as a method of neuroprotection during thoracic aortic surgery. Ann Thorac Surg 2001;72:1774-82.
- Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg 1999;67:1874-8; discussion 1891-4.
- Zierer A, El-Sayed Ahmad A, Papadopoulos N, et al. Selective antegrade cerebral perfusion and mild (28°C-30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg 2012;144:1042-49.
- Swain JA, McDonald TJ Jr, Griffith PK, et al. Low-flow hypothermic cardiopulmonary bypass protects the brain. J Thorac Cardiovasc Surg 1991;102:76-83; discussion 83-4.
- Miyamoto Y, Fukui S, Kajiyama T, et al. Analysis of collateral blood flow to the lower body during selective cerebral perfusion: is three-vessel perfusion better than two-vessel perfusion? Eur J Cardiothorac Surg 2009;35:684-7; discussion 687-8.
- Matalanis G, Shi WY. An Australian experience with aortic arch replacement: a novel approach without circulatory arrest or deep hypothermia. Heart Lung Circ 2011;20:163-9.
- Matalanis G, Koirala RS, Shi WY, et al. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg 2011;142:809-15.
- Bischoff MS, Brenner RM, Scheumann J, et al. Long-term outcome after aortic arch replacement with a trifurcated graft. J Thorac Cardiovasc Surg 2010;140:S71-6; discussion S86-91.
- Carmichael JD. Carotid surgery in the community hospital: 467 consecutive operations. Arch Surg 1980;115:937-9.
- Bland JE, Lazar ML. Carotid endarterectomy without shunt. Neurosurgery 1981;8:153-7.
- Collice M, Arena O, Fontana RA, et al. Role of EEG monitoring and cross-clamping duration in carotid endarterectomy. J Neurosurg 1986;65:815-9.
- Mitchell DG, Merton DA, Graziani LJ, et al. Right carotid artery ligation in neonates: classification of collateral flow with color Doppler imaging. Radiology 1990;175:117-23.
- Lewin JS, Masaryk TJ, Modic MT, et al. Extracorporeal membrane oxygenation in infants: angiographic and parenchymal evaluation of the brain with MR imaging. Radiology 1989;173:361-5.
- Allan JS, Wright CD. Tracheoinnominate fistula: diagnosis and management. Chest Surg Clin N Am 2003;13:331-41.
- Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg 2007;83:S796-8; discussion S824-31.
- Küçüker SA, Ozatik MA, Saritaş A, et al. Arch repair with unilateral antegrade cerebral perfusion. Eur J Cardiothorac Surg 2005;27:638-43.
- Svensson LG, Nadolny EM, Kimmel WA. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2002;74:2040-6.
- Gega A, Rizzo JA, Johnson MH, et al. Multimodal protocol influence on stroke and neurocognitive deficit prevention after ascending/arch aortic operations. Ann Thorac Surg 2007;84:759-66; discussion 766-7.
- Matalanis G, Hata M, Buxton BF. A retrospective comparative study of deep hypothermic circulatory arrest, retrograde, and antegrade cerebral perfusion in aortic arch surgery. Ann Thorac Cardiovasc Surg 2003;9:174-9.
- Gulbins H, Pritisanac A, Ennker J. Axillary versus femoral cannulation for aortic surgery: enough evidence for a general recommendation? Ann Thorac Surg 2007;83:1219-24.
- Kamiya H, Kallenbach K, Halmer D, et al. Comparison of ascending aorta versus femoral artery cannulation for acute aortic dissection type A. Circulation 2009;120:S282-6.
- Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240:529-34; discussion 534.
- Crooke GA, Schwartz CF, Ribakove GH, et al. Retrograde arterial perfusion, not incision location, significantly increases the risk of stroke in reoperative mitral valve procedures. Ann Thorac Surg 2010;89:723-9; discussion 729-30.
- Svensson LG, Blackstone EH, Rajeswaran J, et al. Does the arterial cannulation site for circulatory arrest influence stroke risk? Ann Thorac Surg 2004;78:1274-84; discussion 1274-84.
- Spielvogel D. Aortic arch replacement using a trifurcated graft. Multimedia Manual of Cardio-Thoracic Surgery 2012. 2012 mms002 published online March 28, 2012.
- Etz CD, Plestis KA, Kari FA, et al. Axillary cannulation significantly improves survival and neurologic outcome after atherosclerotic aneurysm repair of the aortic root and ascending aorta. Ann Thorac Surg 2008;86:441-6; discussion 446-7.
- Achneck H, Modi B, Shaw C, et al. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest 2005;128:1580-6.





