Minimally invasive staged segmental artery coil embolization (MIS2ACE) for spinal cord protection
Introduction
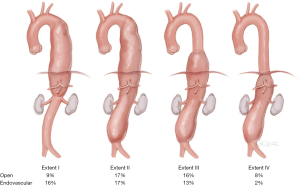
Minimally invasive staged segmental artery coil embolization (MIS2ACE) is an emerging technique currently being investigated in the international, randomized controlled multicenter trial PAPAartis (1), for priming of the paraspinous collateral network in preparation of open or endovascular aortic aneurysm repair (2). The risk of paraplegia in the context of thoracoabdominal aortic aneurysm (TAAA) repair is still high and can be estimated by the extent of the aortic aneurysm according to the Crawford classification (displayed in Figure 1) and the degree of planned repair and is especially relevant for endovascular repair (landing zones!) (2,3).
Several neuroprotective adjuncts have been established in the clinical setting aiming for a reduction in spinal cord ischemia (SCI). Previous experimental and clinical evidence has generated very promising evidence that staging of TAAA repair either by MIS2ACE or by using multiple stent-grafts can significantly reduce the rate of paraplegia (4-6). The pathophysiological background is the presence of a strong paraspinal collateral network and its capacity to remodel by arteriogenesis (7,8). MIS2ACE in the context of TAAA repair has been first described in 2014 (9). One year later the first-in-man cases were performed (10), followed by some larger series (4,11). Since then, MIS2ACE has been thoroughly investigated focusing on technique of coil embolization and choice of occlusion method (12).
Operative techniques
Preoperative planning
Preoperative assessment of the patient includes a contrast-enhanced, thin-layer, computed tomography (CT) of the entire aorta to assess the extent of aortic pathology and to evaluate patency, origin, and diameter of accessible segmental arteries (SA) as well as access options (e.g., femoral). Target SAs for coil deployment are identified considering the extent of planned repair and individual SA anatomy. In case of contraindications to contrast-enhanced CT, magnetic resonance imaging may be used as alternative imaging modality despite less spatial resolution.
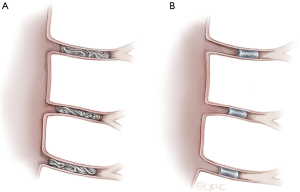
In patients with chronic type B aortic dissection, as displayed in Figure 2, SAs may be already occluded or difficult to access; occasionally entries in the dissection membrane may be considered for access to false-lumen originating SAs. Detailed 3D-reconstruction of the aorta can help plan the procedure and final repair. Especially in endovascular aortic repair, preoperative imaging is essential for stent-graft design and positioning. With regard to aneurysm repair, general assessment of cardiovascular health of the patient is recommended: electrocardiogram (ECG), pulmonary function tests, echocardiogram, and coronary angiogram should all be considered.
Monitoring
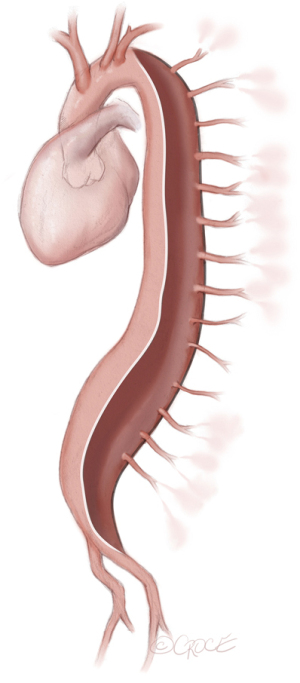
During and after the procedure the patient’s arterial blood pressure is monitored closely (and preferably invasively) for 48 hours. For monitoring of paraspinous muscle oxygenation, near-infrared spectroscopy can be applied as shown in Figure 3 (13). As the patient is awake during the MIS2ACE procedure, neurological assessment can be performed after each SA coil embolization. Caution is advised in case of back pain as sign of ischemia and should mandate interruption of the procedure. The procedure may be performed with or without spinal fluid drainage.
Exposition
SA embolization usually is conducted through a transfemoral access in local anesthesia. The biggest advantage of local instead of general anesthesia is the possibility of immediate feedback by the awake patient with regard to potential neurological symptoms. After access to the common femoral artery, a 6 F angled sheath and a sidewinder-type 5 F catheter is typically used to engage the SA ostium. Then a microcatheter of choice and a hydrophilic guidewire are used to gain a stable access to the main stem of the SA (see Figure 4).
SA occlusion
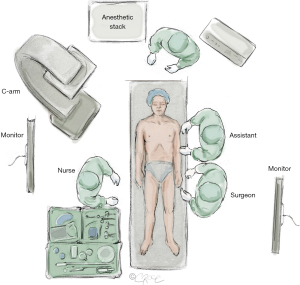
MIS2ACE is usually performed using a fixed imaging system with large flat panel detector in either a hybrid operating room or an angiography suite, overview displayed in Figure 5.
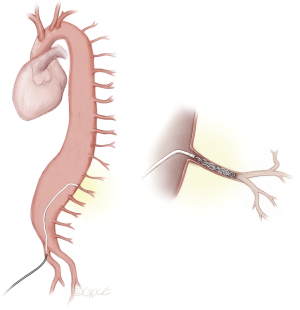
Digital subtraction angiography (DSA) in multiple projections is used to identify SAs. Image-fusion techniques to create an overlay with anatomic information from preoperative CTAs serving as anatomic reference prior to SA catheterization can be very helpful and can reduce radiation and procedure time. SA embolization can be performed using pushable or detachable coils, as well as vascular plugs, as seen in Figure 6.
The use of particles and liquid embolic agents is strongly discouraged, due to the risk of peripheral embolization to the anterior spinal artery. Embolization is aimed to be performed in the proximal SA main stem to ensure that the collateral network itself is not affected. According to the protocol of the ongoing PAPAartis trial, a maximum of seven SAs should be occluded in one MIS2ACE session and a time interval of minimum 21 days between MIS2ACE sessions is recommended, however, a safety minimum of 5 days is possible (1).
Post-MIS2ACE monitoring
It is essential to carefully control the individual patient’s systolic blood pressure during and after the procedure (14). Invasive monitoring of blood pressure is advantageous, and hypotensive periods should be meticulously avoided for a sufficient mean arterial pressure (MAP) and for optimal stimulation of the paraspinous collateral network. Interruption/reduction of oral anti-hypertensive medication and use of intravenous vasopressors is preferable to volume therapy, which increases central venous pressure and thereby cerebrospinal fluid (CSF) pressure, leading to a reduction in spinal cord perfusion (15). Ideally the patient should stay in the intermediate care unit (IMCU) for at least 48 hours. Neurological function should be regularly examined by applying the modified Tarlov scale (16).
Completion
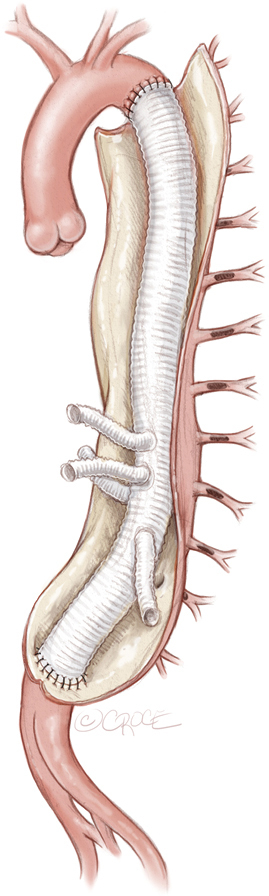
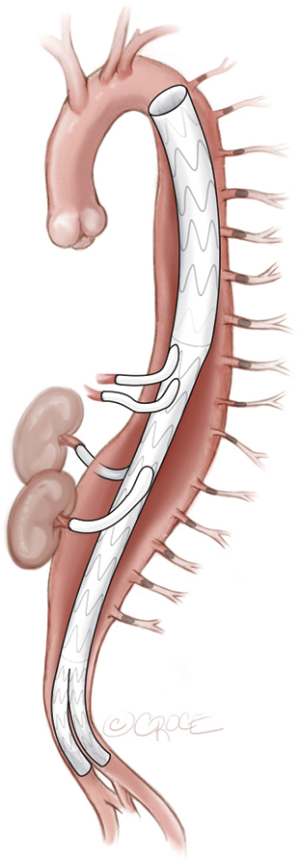
After a minimum of 5 days after the last MIS2ACE procedure, final aneurysm repair can be performed, either as open thoracoabdominal repair (see Figure 7) or endovascular procedure (see Figure 8). MIS2ACE back-bleeding in open repair is reduced and risk of type II endoleak is significantly lower in endovascular repair.
Comments
Clinical results
MIS2ACE has been proven to be an effective tool for reducing the incidence of SCI, by priming the paraspinous collateral network prior to complete thoracoabdominal aortic repair. Large animal experiments, various clinical pilot studies, and numerous retrospective clinical series have shown the benefits of “staging” (6). It is currently being evaluated in a large, international, randomized, controlled multicenter clinical trial (1,4,12). The first preliminary clinical results, gathered before the initiation of the PAPAartis trial, seem promising, and endovascular staging using stent grafts is also already being performed by many leading endo/vascular specialists in the field (5,6,11). The clinical implementation of MIS2ACE was performed in two very high-risk patients—one 45-year-old man receiving MIS2ACE of two unilateral lumbar SAs four weeks prior to open repair of a Crawford Extent III aortic aneurysm, and in one 66-year-old woman receiving bilateral MIS2ACE of the 4th lumbar SA and the inferior mesenteric artery eight weeks prior to endovascular aortic repair of a Crawford Extent II aneurysm (10). Both patients were eventually successfully treated without any signs of neurological impairment. These first two patients were followed by a single-center case series from Branzan et al. including 50 patients, mainly Crawford Extent II and III (72%), receiving MIS2ACE prior to endovascular repair. No neurological deficit occurred, however, 24% of patients developed backpain during the procedure as sign of serious muscle ischemia (4). Recently, Addas et al. presented a retrospective analysis of 17 patients receiving MIS2ACE prior to endovascular repair, also mainly Extent II and III aneurysms. In this analysis, prior SA embolization was successful in 14 patients, and a mean of three arteries were occluded, mainly between T9 and T12 (11). In those patients with MIS2ACE, two patients still developed paraparesis, potentially due to incomplete embolization and therefore insufficient priming of the collateral network (may be due to short interval) or insufficient blood pressure management.
One of the three patients with attempted but unsuccessful MIS2ACE experienced paraplegia. Another case report of insufficient collateral network preparation was published by Doukas et al. involving a 54-year-old man in need for a Crawford Extent II repair after acute type A aortic dissection with ascending and arch replacement, as well as frozen elephant trunk implantation 4 years earlier (17). In three MIS2ACE sessions, a total of eight SAs were occluded (T8 and T9 right sided, T11, L1 and L2 bilateral). However, during open repair, a significant decrease of motor-evoked potentials (MEPs) occurred and the surgeons found two small SAs at T12, which then were revascularized with an 8-mm polyester graft, and MEPs were reported to have been restored. The patient was discharged with no neurological deficits (17). This case demonstrates the know-how and expertise needed for sufficient occlusion of all available SAs, especially in the highly relevant, so called ‘watershed area’, between T12 and L2, prior to the repair as demonstrated in a chronic large animal experiment on the optimal occlusion pattern (12).
Advantages
The most significant advantage of MIS2ACE, if confirmed by the ongoing PAPAartis trial, will be the reduction of paraplegia in the context of TAAA repair. The technique itself is easy to learn, however, is complicated by anatomical features of the individual patients. Up to now, no severe procedural complications have been reported by over 33 centers and over 150 MIS2ACE procedures (4,11) and with advancing learning curve the amount of contrast agent and time of radiation is reduced. Furthermore, as the patient is awake, neurological symptoms, such as ischemic back pain or symptoms of sudden paralysis, can be reported immediately and the procedure can be interrupted. In contrast to staging by stenting, which should be applied cautiously as the regenerative potential of the paraspinous collateral network is unknown for the individual patient, and once the stent-graft is deployed and several SAs are occluded simultaneously, there is no option of reversal. One underestimated advantageous side-effect of MIS2ACE is the reduction of back-bleeding. Segmental back-bleeding after opening of the aneurysm can worsen the overview of the operative field, and therefore back-bleeding can be a severe threat to the spinal cord as shown by an experimental study of our group (18). In endovascular repair, previous MIS2ACE can reduce the incidence of type II endoleaks.
Caveats
However, MIS2ACE is neither a diagnostic nor a therapeutic procedure itself. Therefore, safety of the patient has priority. In patients with a shaggy aorta, MIS2ACE should be considered cautiously due to an increased embolic risk. Also, there is no room for MIS2ACE in the case of emergency treatment of the aorta. In all patients, kidney and thyroid function requires testing due to the use of iodinated contrast agents. Furthermore, the individual timing for consecutive MIS2ACE and the final repair remains to be investigated and depends on the individual regenerative potential of the paraspinal collateral network. Patient-associated risk factors, like age, sarcopenia, and other co-morbidities are not only relevant for the success of the final repair, but also for priming of the paraspinous collateral network by MIS2ACE, since it is located in the paraspinal muscles (19). A recent study by Chatterjee et al. on 392 patients aged 60 years or older receiving open TAAA repair revealed that sarcopenia did not influence early mortality or midterm survival but was—not surprisingly—associated with an increased risk for permanent paraplegia [odds ratio (OR) =3.29, P=0.01] (19).
All other previously established neuroprotective adjuncts should be continued to be applied peri- and postoperatively (i.e., CSF drainage, permissive hypertension, sufficient hematocrit, monitoring of spinal cord oxygenation with collateral network) according to the individual patient. Maurel et al. demonstrated a ten-fold reduction in paraplegia rate from 14% to 1.2% by early restoration of blood flow via withdrawal of large sheaths and by optimizing the perioperative protocol (20), suggesting that safeguarding inflow to the collateral network, particularly during the perioperative period, is crucial. Any obstruction of large collateral network inflow vessels has to be avoided.
MIS2ACE and ‘staging’ are promising techniques in reducing the incidence of permanent spinal cord injury, by priming of the paraspinous collateral network and maintaining patient mobility and quality of life, thereby reducing long-term mortality. The technique is safe and may be trained on a vascular simulator. The learning curve is steep, leading to a reduction in amount of contrast agent and radiation time.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petroff D, Czerny M, Kölbel T, et al. Paraplegia prevention in aortic aneurysm repair by thoracoabdominal staging with 'minimally invasive staged segmental artery coil embolisation' (MIS2ACE): trial protocol for a randomised controlled multicentre trial. BMJ Open 2019;9:e025488. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2016;151:1323-37. [Crossref] [PubMed]
- Katsargyris A, Oikonomou K, Kouvelos G, et al. Spinal cord ischemia after endovascular repair of thoracoabdominal aortic aneurysms with fenestrated and branched stent grafts. J Vasc Surg 2015;62:1450-6. [Crossref] [PubMed]
- Branzan D, Etz CD, Moche M, et al. Ischaemic preconditioning of the spinal cord to prevent spinal cord ischaemia during endovascular repair of thoracoabdominal aortic aneurysm: first clinical experience. EuroIntervention 2018;14:828-35. [Crossref] [PubMed]
- Etz CD, Zoli S, Mueller CS, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2010;139:1464-72. [Crossref] [PubMed]
- Dias-Neto M, Tenorio ER, Huang Y, et al. Comparison of single- and multistage strategies during fenestrated-branched endovascular aortic repair of thoracoabdominal aortic aneurysms. J Vasc Surg 2023;77:1588-1597.e4. [Crossref] [PubMed]
- Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg 2011;141:1020-8. [Crossref] [PubMed]
- Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice. J Thorac Cardiovasc Surg 2011;141:1029-36. [Crossref] [PubMed]
- Luehr M, Salameh A, Haunschild J, et al. Minimally invasive segmental artery coil embolization for preconditioning of the spinal cord collateral network before one-stage descending and thoracoabdominal aneurysm repair. Innovations (Phila) 2014;9:60-5. [Crossref] [PubMed]
- Etz CD, Debus ES, Mohr FW, et al. First-in-man endovascular preconditioning of the paraspinal collateral network by segmental artery coil embolization to prevent ischemic spinal cord injury. J Thorac Cardiovasc Surg 2015;149:1074-9. [Crossref] [PubMed]
- Addas JAK, Mafeld S, Mahmood DN, et al. Minimally Invasive Segmental Artery Coil Embolization (MISACE) Prior to Endovascular Thoracoabdominal Aortic Aneurysm Repair. Cardiovasc Intervent Radiol 2022;45:1462-9. [Crossref] [PubMed]
- von Aspern K, Haunschild J, Simoniuk U, et al. Optimal occlusion pattern for minimally invasive staged segmental artery coil embolization in a chronic porcine model. Eur J Cardiothorac Surg 2019;56:126-34. [Crossref] [PubMed]
- Etz CD, von Aspern K, Gudehus S, et al. Near-infrared spectroscopy monitoring of the collateral network prior to, during, and after thoracoabdominal aortic repair: a pilot study. Eur J Vasc Endovasc Surg 2013;46:651-6. [Crossref] [PubMed]
- Etz CD, Di Luozzo G, Zoli S, et al. Direct spinal cord perfusion pressure monitoring in extensive distal aortic aneurysm repair. Ann Thorac Surg 2009;87:1764-73; discussion 1773-4. [Crossref] [PubMed]
- Haunschild J, von Aspern K, Khachatryan Z, et al. Detrimental effects of cerebrospinal fluid pressure elevation on spinal cord perfusion: first-time direct detection in a large animal model. Eur J Cardiothorac Surg 2020;58:286-93. [Crossref] [PubMed]
- Chiesa R, Melissano G, Marrocco-Trischitta MM, et al. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 2005;42:11-7. [Crossref] [PubMed]
- Doukas P, Gombert A, Kotelis D, et al. Motor evoked potential-guided segmental artery revascularization during open thoracoabdominal aortic aneurysm surgery after coil embolization as a part of the minimally invasive staged segmental artery coil embolization concept. J Vasc Surg Cases Innov Tech 2022;8:206-9. [Crossref] [PubMed]
- Haunschild J, von Aspern K, Herajärvi J, et al. Impact of distal aortic perfusion on 'segmental steal' depleting spinal cord blood flow-a quantitative experimental approach. Eur J Cardiothorac Surg 2022;62:ezac213. [Crossref] [PubMed]
- Chatterjee S, Shi A, Yoon L, et al. Effect of sarcopenia on survival and spinal cord deficit outcomes after thoracoabdominal aortic aneurysm repair in patients 60 years of age and older. J Thorac Cardiovasc Surg 2023;165:1985-1996.e3. [Crossref] [PubMed]
- Maurel B, Delclaux N, Sobocinski J, et al. The impact of early pelvic and lower limb reperfusion and attentive peri-operative management on the incidence of spinal cord ischemia during thoracoabdominal aortic aneurysm endovascular repair. Eur J Vasc Endovasc Surg 2015;49:248-54. [Crossref] [PubMed]