Surgical ventricular reconstruction for ischemic cardiomyopathy—a systematic review and meta-analysis of 7,685 patients
Introduction
With an ever-increasing disease burden, coronary artery disease is the foremost cause of heart failure and myocardial infarction (1). Acute myocardial infarction (AMI) leads to a number of acute and chronic complications that are mediated by various factors. Post-AMI sequelae are characterized by myocardial remodeling—an umbrella term for repair of necrotic heart muscle through thinning, formation of collagen scars, compensatory hypertrophy of non-infarcted myocardium, and eventual ventricular dilation with change in its geometry (2,3). The mechanism underlying remodeling is change at a molecular and cellular level which occurs in response to myocardial necrosis post-AMI. Maladaptive remodeling further negatively affects ventricular function and eventually leads to congestive heart failure, the incidence of which is increasing in survivors of acute MI. Current therapies are targeted at preventing and reversing such adverse remodeling (1-3).
Surgical ventricular reconstruction (SVR) is an attempt at controlling adverse ventricular remodeling by excluding dyskinetic and scarred ventricle, prevention of further ventricular dilation, and restoration of the geometry of the ventricle (2). This procedure, with its subsequent modifications, was pioneered by Cooley (4), Dor (5) and Jatene (6). However, the Dor procedure/modification is the most widely used technique of SVR, which is otherwise called endoventricular patch plasty (EVCPP) (7). The aim is to reduce LV volume and restore the elliptical shape of the ventricle thereby improving EF as well as cardiac function. This surgery is usually undertaken concurrently with coronary artery bypass graft (CABG) surgery.
In 2009, results of the Surgical Treatment for Ischemic Heart Failure (STICH) trial (8) reported greater reduction in ventricular volume with addition of SVR to CABG but no difference in rates of death or hospitalization due to cardiac causes. These results put into question the utility of SVR when combined with CABG and led to a decrease in its popularity (2,9). Being the most consequential trial on SVR efficacy in the last few decades, the STICH trial started conversations regarding the proper place of SVR in treatment of post-MI remodeling and heart failure.
Given the discordant views and results regarding the efficacy of SVR, we sought to gather and analyze in granular detail, all available evidence on the utilization of SVR in patients suffering from ischemic cardiomyopathy. Through this systematic review and meta-analysis, we hope to evaluate the efficacy and utility of SVR.
Methods
Literature search strategy
An electronic database search was performed in June 2020 using MEDLINE (Ovid SP), Scopus, Cochrane Controlled Trials Register (CCTR) and Cumulative Index to Nursing and Allied Health Literature (CINAHL). To achieve maximum sensitivity, the following terms were combined: “Dor procedure”, “modified Dor”, “endoventricular circular patch plasty”, “endoventricular plasty”, “Endoventricular patch plasty”, “endocardial patch”, “ventricular reconstruction”, “ventricular restoration”, “Surgical ventricular restoration”, “ventricular infarct exclusion”, “ventricular aneurysmectomy”, “Ventricular aneurysm repair”, “ventricular endocardial restoration” and “Ventriculoplasty” as either key words or MeSH terms. The references of included studies were manually searched (BF, GG) and did not yield additional studies for inclusion.
Eligibility criteria
Eligible articles for this systematic review were full-length studies published from January 2000 to May 2020 in the English literature that included adults undergoing left ventricular reconstruction for ischemic cardiomyopathy. All technical approaches for SVR were included. Studies that included patients not undergoing left ventricular reconstruction or included patients with non-ischemic cardiomyopathy were excluded. Case reports, abstracts, conference presentations, editorials, reviews and expert opinions were also excluded. When institutions published more than one study including overlapping patient populations, only the most complete reports were included. The Newcastle-Ottawa Scale (NOS) scoring system was utilized to assess risk of bias for the identified studies as recommended by the Cochrane Collaboration.
Data extraction and critical appraisal
Study level data were extracted from the text, figures and tables of all eligible articles (BF, KM, GG). Discrepancies between the reviewers were resolved by discussion and consensus.
Statistical analysis
Baseline patient characteristics and clinical outcomes were reported as the pooled mean with 95% confidence intervals (CI). For dichotomous variables, a meta-analysis of proportions with logit transformation was conducted for preoperative and postoperative variables. Continuous data were combined via meta-analysis with random-effects model. Heterogeneity was evaluated using Cochran Q and I2 test. Survival data from each study were collected and pooled to retrieve a weighted mean and 95% confidence interval at specific time points. Such data were then graphically displayed to visualize survival over time. R software 3.5.0, meta package (R Foundation for Statistical Computing, Vienna, Austria) was used for data analysis. P values <0.05 were considered statistically significant.
Results
Study characteristics
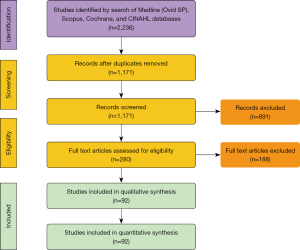
A total of 2,236 articles were identified by conducting the search of four databases. Eligible studies included all prospective and retrospective studies on patients who underwent SVR for ischemic cardiomyopathy. After removal of duplicate articles, 1,171 articles remained. A total of 891 articles were excluded after a detailed evaluation of the title and abstract of each article. The remaining 280 articles underwent a full text evaluation, of which ninety-two articles met inclusion criteria with a collective 7,685 patients. The mean NOS score for included studies was six. Sixty studies had a score of either six or seven, indicating that a majority of the included studies were of fair to good quality. A PRISMA flow diagram illustrating the search strategy is provided in Figure 1. A detailed list of the studies included is located in the supplementary material as Table S1. In addition, NOS scoring details for all included studies are presented in the supplementary material as Table S2.
Baseline characteristics
The mean age of patients was 61 years (95% CI: 59–63) with 80% (78–82%) being male. Common comorbidities included hypertension [56% (52–61%)], hyperlipidemia [52% (45–58%)], and diabetes [30% (27–32%)]. Congestive heart failure was present in 66% (54–78%) while angina was seen in 58% (45–70%) of patients at time of presentation. Akinesis of ventricular wall segments was seen in 46% (31–61%) while 54% (36–71%) of patients presented with dyskinesis. Average time from previous myocardial infarction to surgery was 18.2 months (9.4–27.0) and 25% (18–33%) of patients had undergone previous percutaneous coronary intervention (PCI). The mean number of coronary vessels involved was 2.5 (2–3). Left anterior descending disease was most frequently seen [93% (88–97%)] followed by right coronary [58% (37–80%)], and left circumflex [45% (11–79%)] disease respectively. Further baseline characteristics are shown in Table 1.
Table 1
| Characteristics | Pooled value [95% CI] | No. of studies | No. of patients (N or n/N) | I2 (%) |
|---|---|---|---|---|
| Age (years) | 61.1 [59.4, 62.8] | 72 | 6,268 | 100 |
| Male (%) | 80 [78, 82] | 78 | 5,254/6,647 | 81* |
| BMI (kg/m2) | 25.7 [23.4, 27.9] | 7 | 1,000 | 0 |
| BSA (m2) | 1.7 [1.6, 1.8] | 7 | 232 | 0 |
| Hypertension (%) | 56 [52, 61] | 40 | 2,092/3,957 | 86* |
| Hyperlipidemia (%) | 52 [45, 58] | 22 | 1,083/2,122 | 88* |
| Pulmonary hypertension (%) | 23 [13, 32] | 5 | 85/366 | 84* |
| Angina (%) | 58 [45, 70] | 25 | 1,539/3,038 | 99* |
| Unstable angina (%) | 34 [21, 48] | 5 | 104/353 | 88* |
| Cerebrovascular disease (%) | 12 [8, 15] | 7 | 77/595 | 46 |
| Chronic lung disease (%) | 17 [12, 22] | 13 | 186/998 | 87* |
| Smoker, previous or current (%) | 58 [52, 64] | 22 | 1,288/2,160 | 90* |
| Diabetes (%) | 30 [27, 32] | 54 | 1,450/4,995 | 78* |
| Atrial fibrillation (%) | 14 [10, 18] | 11 | 214/1,750 | 87* |
| Ventricular tachycardia (%) | 22 [13, 30] | 10 | 263/994 | 94* |
| Congestive heart failure (%) | 66 [54, 78] | 12 | 848/1,258 | 99* |
| Renal dysfunction (%) | 10 [6, 14] | 17 | 232/1,443 | 90* |
| Peripheral vascular disease (%) | 10 [8, 11] | 14 | 124/1,150 | 11 |
| Previous heart surgery (%) | 6 [3, 9] | 12 | 80/865 | 79* |
| Previous ICD implant (%) | 12 [7, 18] | 5 | 90/575 | 72* |
| Previous PCI (%) | 25 [18, 33] | 13 | 324/1,212 | 90* |
| Previous MI (%) | 100 [99, 100] | 65 | 5,509/5,637 | 63* |
| Previous MI, anterior (%) | 99 [98, 100] | 16 | 1,225/1,274 | 73* |
| Previous MI, posterior or inferior (%) | 16 [8, 24] | 6 | 60/330 | 82* |
| Previous MI, anteroseptal (%) | 87 [79, 94] | 6 | 509/602 | 99* |
| Time from previous MI to surgery (months) | 18.2 [9.4, 27.0] | 7 | 1,915 | 65* |
| Aneurysm location | ||||
| Anterior (%) | 92 [89, 95] | 20 | 1,558/1,741 | 84* |
| Anteroseptal (%) | 77 [70, 84] | 4 | 398/468 | 98* |
| Apico-anterior (%) | 89 [81, 97] | 4 | 332/379 | 89* |
| Inferior (%) | 5 [2, 9] | 4 | 22/316 | 44 |
| Posterior (%) | 18 [7, 28] | 10 | 125/1,122 | 98* |
| Thrombus in left ventricle (%) | 38 [26, 51] | 12 | 341/921 | 95* |
| Coronary vessel disease, No. of vessels (mean) | 2.5 [2.0, 3.1] | 6 | 357 | 0 |
| Single vessel disease (%) | 14 [11, 18] | 29 | 547/3,020 | 89* |
| Double vessel disease (%) | 23 [19, 26] | 28 | 732/2,878 | 79* |
| Triple vessel disease (%) | 60 [54, 66] | 33 | 1,828/3,346 | 93* |
| Left main disease (%) | 10 [8, 13] | 22 | 250/2,151 | 66* |
| Left anterior descending artery disease (%) | 93 [88, 97] | 10 | 698/780 | 89* |
| Left circumflex artery disease (%) | 45 [11, 79] | 4 | 73/159 | 97* |
| Right coronary artery disease (%) | 58 [37, 80] | 4 | 86/132 | 87* |
| LV akinesis (%) | 46 [31, 61] | 10 | 476/1,178 | 97* |
| LV dyskinesis (%) | 54 [36, 71] | 10 | 674/1,294 | 98* |
*, heterogeneity P<0.05 (significant data heterogeneity present). BMI, body mass index; BSA, body surface area; ICD, implantable cardioverter defibrillator; PCI, percutaneous coronary intervention; MI, myocardial Infarction; LV, left ventricle.
Pre-operative characteristics
At time of surgery, mean LVEF was 30.0% (28.8–31.2%) with a LVEDVI of 119.9 (112.1–127.6) mL/m2 and a LVESVI of 88.2 (81.6–94.8) mL/m2 respectively. Mean MR grade was 1.8 (1.4–2.1) with an associated New York Heart Association (NYHA) class of 3.0 (2.8–3.1). 32% (21–42%) of patients were on inotropic support and 15% (4–25%) required an intra-aortic balloon pump (IABP). Additional pre-operative characteristics are included in Table 2.
Table 2
| Characteristics | Pooled value [95% CI] | No. of studies | No. of patients (N or n/N) | I2 (%) |
|---|---|---|---|---|
| EF (%) | 30.0 [28.8, 31.2] | 82 | 6,925 | 0 |
| Cardiac Index (L/min/m2) | 2.4 [2.2, 2.6] | 10 | 583 | 0 |
| Stroke volume index (mL/m2) | 30.1 [23.0, 38.9] | 3 | 168 | 0 |
| LVEDVI (mL/m2) | 119.9 [112.1, 127.6] | 45 | 3,325 | 15 |
| LVESVI (mL/m2) | 88.2 [81.6, 94.8] | 37 | 3,723 | 62* |
| LVEDV (mL) | 200.8 [174.6, 227.0] | 16 | 1,095 | 55* |
| LVESV (mL) | 128.9 [105.3, 152.5] | 16 | 1,076 | 71* |
| LVEDD (mm) | 63.8 [62.0, 65.6] | 36 | 3,267 | 0 |
| LVESD (mm) | 49.9 [48.1, 51.7] | 29 | 2,275 | 0 |
| Diastolic eccentricity index | 0.8 [0.7, 0.8] | 6 | 541 | 0 |
| Systolic eccentricity index | 0.8 [0.8, 0.9] | 4 | 434 | 0 |
| Diastolic sphericity index | 0.6 [0.6, 0.7] | 6 | 1,171 | 60* |
| Systolic Sphericity index | 0.6 [0.6, 0.7] | 4 | 717 | 0 |
| E/A (cm/s) | 2.7 [0.5, 4.8] | 7 | 738 | 93* |
| E/E' (cm/s) | 18.7 [11.0, 26.3] | 4 | 199 | 0 |
| Decel time (ms) | 161.7 [135.9, 187.6] | 6 | 320 | 0 |
| Pulmonary artery pressure (mmHg) | 29.9 [25.3, 32.6] | 16 | 1,678 | 0 |
| Pulmonary capillary wedge pressure (mmHg) | 13.6 [10.1, 17.1] | 7 | 467 | 0 |
| MR Grade (mean) | 1.8 [1.4, 2.1] | 17 | 1127 | 39* |
| MR Grade 0 (%) | 27 [18, 37] | 13 | 339/1,321 | 97* |
| MR Grade 1 (%) | 32 [24, 39] | 18 | 486/1,724 | 96* |
| MR Grade 2 (%) | 22 [16, 27] | 17 | 364/1,582 | 90* |
| MR Grade 3 (%) | 11 [6, 15] | 15 | 287/1,813 | 92* |
| MR Grade 4 (%) | 6 [4, 8] | 17 | 154/1,634 | 87* |
| NYHA Class (mean) | 3.0 [2.8, 3.1] | 34 | 2,092 | 0 |
| NYHA Class I (%) | 1 [0, 2] | 34 | 87/2,656 | 57* |
| NYHA Class II (%) | 22 [18, 27] | 35 | 655/2,757 | 96* |
| NYHA Class III (%) | 51 [45, 56] | 40 | 1,676/3,347 | 90* |
| NYHA Class IV (%) | 22 [17, 26] | 42 | 813/3,436 | 94* |
| Inotropic support (%) | 32 [21, 42] | 8 | 150/403 | 83* |
| IABP (%) | 15 [4, 25] | 15 | 106/822 | 97* |
| Urgent/emergent procedure (%) | 13 [9, 16] | 23 | 1,684 | 85* |
*, heterogeneity P<0.05 (significant data heterogeneity present). LVEDVI, left ventricle end-diastolic volume index; LVESVI, left ventricle end-systolic volume index; LVEDV, left ventricle end-diastolic volume; LVESV, left ventricle end-systolic volume; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; MR, mitral regurgitation; NYHA, New York Heart Association; IABP, intra-aortic balloon pump.
Operative characteristics
The mean cardiopulmonary bypass time was 128 minutes (117–140 minutes) and the aortic cross-clamp time was 84.5 minutes (76–93 minutes). Concurrent with SVR, 92% (90–93%) of patients underwent CABG with an average of 2.6 grafts [2.3–2.9]. Other concomitant procedures included cryoablation [26% (17–35%)], mitral valve repair [21% (18–24%)] and tricuspid valve repair/replacement [14% (10–19%)]. Utilization of a ventricular patch [67% (58–76%)] was preferred to performing SVR without a patch [33% (24–42%)]. Further intraoperative details are presented in Table 3.
Table 3
| Pooled value [95% CI] | No. of studies | No. of patients (N or n/N) | I2 (%) | |
|---|---|---|---|---|
| CPB (%) | 99 [98, 100] | 71 | 5,272/5,318 | 98* |
| CPB time (minutes) | 128.2 [116.6, 139.8] | 42 | 3,487 | 68* |
| Aortic cross clamp (%) | 91 [86, 97] | 43 | 2,910/3,225 | 100* |
| Aortic cross clamp time (minutes) | 84.5 [76.0, 93.0] | 37 | 3,014 | 28* |
| Cardioplegia (%) | 86 [81, 91] | 56 | 3,368/3,838 | 100* |
| SVR technical approach | ||||
| Patch (%) | 67 [58, 76] | 84 | 3,737/6,885 | 100* |
| Dor/circular patch (%) | 95 [91, 97] | 45 | 2,552/3,522 | 92* |
| Patchless (%) | 33 [24, 42] | 84 | 3,139/6,885 | 100* |
| Linear (%) | 81 [78, 85] | 23 | 1,817/2,268 | 98* |
| Balloon mannequin utilized (%) | 92 [72, 100] | 16 | 1,328/1,633 | 100* |
| Concomitant procedure | ||||
| CABG (%) | 92 [90, 93] | 82 | 5,950/6,817 | 91* |
| No. of grafts (mean) | 2.6 [2.3, 2.9] | 45 | 3,764 | 0 |
| No. of grafts: 1 (%) | 19 [12, 25] | 10 | 119/543 | 74* |
| No. of grafts: 2 (%) | 24 [15, 33] | 8 | 85/311 | 70* |
| No. of grafts: 3 (%) | 28 [17, 40] | 8 | 107/311 | 81* |
| No. of grafts: >3 (%) | 18 [10, 26] | 6 | 43/226 | 58* |
| Internal mammary artery harvested (%) | 67 [57, 76] | 27 | 1,847/2,868 | 99* |
| LAD revascularized (%) | 84 [79, 90] | 11 | 951/1,123 | 87* |
| Aortic valve replacement or repair (%) | 2 [1, 4] | 11 | 37/1,282 | 33 |
| Mitral valve repair (%) | 21 [18, 24] | 70 | 1,046/5,426 | 99* |
| Mitral valve replacement (%) | 1 [0, 1] | 43 | 73/3,429 | 19 |
| Tricuspid valve replacement / repair (%) | 14 [10, 19] | 14 | 186/1,386 | 91* |
| Cryoablation (%) | 26 [17, 35] | 13 | 419/1,440 | 96* |
| Ventricular septal defect closure (%) | 3 [2, 5] | 7 | 31/737 | 23 |
*, heterogeneity P<0.05 (significant data heterogeneity present). CPB, cardiopulmonary bypass; SVR, surgical ventricular reconstruction; CABG, coronary artery bypass graft; LAD, left anterior descending.
Pre vs. post-operative comparison
There was a significant increase in LVEF after SVR [pre-operative, 30.0% (28.8–31.2%) vs. post-operative, 40.9% (39.4–42.4%), P<0.01] along with decrease in NYHA class [pre-operative, 3.0 (2.8–3.1) vs. post-operative, 1.8 (1.5–2.0), P<0.01]. There were significant decreases after SVR in LVESVI [pre-operative, 83.9 (79.3–88.4) mL/m2vs. post-operative, 46.8 (43.5–50.1) mL/m2, P<0.01] and LVEDVI [pre-operative, 111.9 (112.1–127.6) mL/m2vs. post-operative, 79.6 (73.6–85.7) mL/m2, P<0.01]. The incidence of pulmonary hypertension trended down from 23% (14–37%) preoperatively to 15% (5–40%) postoperatively (P=0.34). Pulmonary capillary wedge pressure (PCWP) was comparable pre vs. post-SVR [13.6 (10.1–17.1) mmHg vs. 10.9 (7.5–14.2) mmHg, P=0.27]. Further post-operative details are presented in Table 4, and pre- to post-operative comparisons are included in Table 5.
Table 4
| Pooled value [95% CI] | No. of studies | No. of patients (N or n/N) | I2 (%) | |
|---|---|---|---|---|
| Latest follow-up EF (%) | 40.9 [39.4, 42.4] | 63 | 5,310 | 0 |
| EF, 3 month (%) | 39.0 [34.9, 43.1] | 4 | 250 | 0 |
| EF, 1 year (%) | 41.1 [35.0, 47.3] | 6 | 408 | 0 |
| EF, 2 year (%) | 37.8 [32.3, 43.4] | 5 | 395 | 0 |
| Cardiac index (L/min/m2) | 2.8 [2.5, 3.2] | 6 | 373 | 0 |
| SVI (mL/m2) | 29.2 [18.1, 40.2] | 5 | 222 | 34 |
| LVEDVI (mL/m2) | 79.6 [73.6, 85.7] | 35 | 2,363 | 14 |
| LVESVI (mL/m2) | 46.8 [43.5, 50.1] | 40 | 2,747 | 0 |
| LVEDV (mL) | 140.1 [121.1, 159.1] | 18 | 1,596 | 59* |
| LVESV (mL) | 72.2 [60.5, 83.9] | 17 | 1,438 | 40* |
| LVEDD (mm) | 58.3 [56.6, 60.0] | 30 | 2,884 | 0 |
| LVESD (mm) | 45.0 [42.8, 47.3] | 22 | 1,897 | 0 |
| Diastolic eccentricity index | 0.7 [0.7, 0.8] | 6 | 541 | 29 |
| Systolic eccentricity index | 0.8 [0.7, 0.9] | 4 | 434 | 0 |
| Diastolic sphericity index | 0.6 [0.5, 0.7] | 5 | 1,028 | 70* |
| Systolic sphericity index | 0.6 [0.6, 0.7] | 3 | 574 | 28 |
| E/A (cm/s) | 1.7 [0.9, 2.4] | 5 | 576 | 43 |
| E/E' (cm/s) | 21.2 [14.0, 28.4] | 3 | 180 | 0 |
| Pulmonary artery pressure, mean (mmHg) | 25.6 [22.2, 29.0] | 8 | 542 | 0 |
| Pulmonary capillary wedge pressure (mmHg) | 10.9 [7.5, 14.2] | 6 | 352 | 0 |
| Pulmonary hypertension (%) | 15 [5, 25] | 3 | 34/208 | 82* |
| MR Grade (mean) | 0.9 [0.7, 1.1] | 14 | 1,028 | 0 |
| MR Grade 0 (%) | 43 [29, 57] | 7 | 532/1,070 | 88* |
| MR Grade 1 (%) | 22 [12, 38] | 8 | 206/1,114 | 92* |
| MR Grade 2 (%) | 14 [8, 24] | 7 | 229/1,025 | 86* |
| MR Grade 3 (%) | 6 [3, 10] | 5 | 19/393 | 0 |
| MR grade 4 (%) | 3 [2, 5] | 5 | 15/455 | 0 |
| NYHA Class (mean) | 1.8 [1.5, 2.0] | 21 | 1,181 | 0 |
| NYHA Class I (%) | 49 [36, 62] | 23 | 798/1,916 | 98* |
| NYHA Class II (%) | 31 [23, 39] | 22 | 499/1,632 | 93* |
| NYHA Class III (%) | 6 [4, 8] | 20 | 99/1,306 | 71* |
| NYHA Class IV (%) | 1 [0, 2] | 19 | 28/1,254 | 6 |
*, heterogeneity P<0.05 (significant data heterogeneity present). EF, ejection fraction; SVI, systolic volume index; LVEDVI, left ventricle end-diastolic volume index; LVESVI, left ventricle end-systolic volume index; LVEDV, left ventricle end-diastolic volume; LVESV, left ventricle end-systolic volume; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; MR, mitral regurgitation; NYHA, New York Heart Association.
Table 5
| Characteristics | Pre-operative | Post-operative | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of patients | Pooled value [95% CI] | I2 | No. of studies | No. of patients | Pooled value [95% CI] | I2 | |||
| EF (%) | 82 | 6,925 | 30.0 [28.8, 31.2] | 0 | 63 | 5,310 | 40.9 [39.4, 42.4] | 0 | <0.01 | |
| Cardiac index (L/min/m2) | 10 | 583 | 2.4 [2.2, 2.6] | 0 | 6 | 373 | 2.8 [2.5, 3.2] | 0 | 0.04 | |
| LVEDVI (mL/m2) | 45 | 3,325 | 119.9 [112.1, 127.6] | 15 | 35 | 2,363 | 79.6 [73.6, 85.7] | 14 | <0.01 | |
| LVESVI (mL/m2) | 51 | 3,723 | 83.9 [79.3, 88.4] | 0 | 40 | 2,747 | 46.8 [43.5, 50.1] | 0 | <0.01 | |
| LVEDD (mm) | 36 | 3,267 | 63.8 [62.0, 65.6] | 0 | 30 | 2,884 | 58.3 [56.6, 60.0] | 0 | <0.01 | |
| LVESD (mm) | 29 | 2,275 | 49.9 [48.1, 51.7] | 0 | 22 | 1,897 | 45.0 [42.8, 47.3] | 0 | <0.01 | |
| PAP (mmHg) | 16 | 1,678 | 29.0 [25.3, 32.6] | 0 | 8 | 542 | 25.6 [22.2, 29.0] | 0 | 0.18 | |
| PCWP (mmHg) | 7 | 467 | 13.6 [10.1, 17.1] | 0 | 6 | 352 | 10.9 [7.5, 14.2] | 0 | 0.27 | |
| Pulmonary hypertension (%) | 5 | 85/366 | 23 [13, 32] | 84* | 3 | 34/208 | 15 [5, 25] | 82* | 0.34 | |
| MR grade (mean) | 17 | 1,127 | 1.6 [1.3, 1.8] | 16 | 14 | 1,028 | 0.9 [0.7, 1.1] | 0 | <0.01 | |
| NYHA class (mean) | 34 | 2,092 | 3.0 [2.8, 3.1] | 0 | 21 | 1,181 | 1.8 [1.5, 2.0] | 0 | <0.01 | |
*, heterogeneity P<0.05 (significant data heterogeneity present). EF, Ejection fraction; LVEDVI, left ventricle end-diastolic volume index; LVESVI, left ventricle end-systolic volume index; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end-systolic diameter; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; MR, mitral regurgitation; NYHA, New York Heart Association.
Survival and outcomes
Mean total hospital length of stay was twelve days (10–15) while ICU length of stay was four days (3.3–5.5). The 30-day mortality was 4% (3–5%) while late mortality was 19% (9–34%) at a mean follow-up of 27.5 [21–34] months. There was no difference in total mortality between the Dor [12% (8–17%)] and linear techniques [20% (7–44%)] (P=0.16). Figure 2 shows cumulative survival up to 15 years post SVR. The most frequent postoperative complications included atrial fibrillation [23% (17–29%)], low cardiac output [13% (9–17%)] and infection [11% (5–18%)]. The most frequent causes of death were heart failure [7% (5–10%)], arrhythmia [3% (1–4%)] and sepsis [3% (1–5%)]. Seven percent (3–11%) of patients required subsequent placement of a pacemaker and 2% (0–5%) required a left ventricular assist device (LVAD). Further outcomes and complications are described in Table 6.
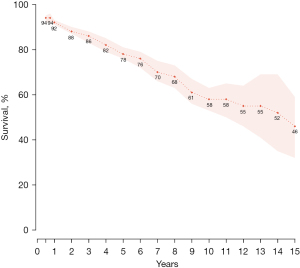
Table 6
| Pooled value [95% CI] | No. of studies | No. of patients (N or n/N) | I2 (%) | |
|---|---|---|---|---|
| Hospital admission | ||||
| Prolonged ventilation (%) | 9 [4, 14] | 6 | 34/341 | 65* |
| Time on ventilator (hours) | 27.0 [20.9, 33.1] | 6 | 369 | 0 |
| Placement of IABP intra- or post-operative (%) | 20 [16, 24] | 50 | 864/4,611 | 95* |
| Inotropic support required (%) | 43 [33, 53] | 14 | 766/1,663 | 95* |
| Inotropic support duration (hours) | 44.2 [22.0, 66.5] | 3 | 175 | 0 |
| Hospital length of stay (days) | 12.4 [9.8, 15.0] | 13 | 821 | 18 |
| ICU length of stay (days) | 4.4 [3.3, 5.5] | 13 | 974 | 0 |
| Total follow up (months) | 27.5 [21.0, 34.0] | 24 | 2,201 | 59* |
| Mortality | ||||
| Operative mortality (%) | 4 [3, 5] | 74 | 344/6,414 | 48* |
| Late mortality (%) | 19 [9, 34] | 8 | 122/577 | 80* |
| Mortality, Linear (%) | 20 [7, 44] | 5 | 118/464 | 82* |
| Mortality, Dor (%) | 12 [8, 17] | 10 | 42/399 | 20 |
| Subsequent procedure | ||||
| Placement of defibrillator (%) | 5 [3, 8] | 9 | 67/1,080 | 65* |
| Placement of pacemaker (%) | 7 [3, 11] | 3 | 48/873 | 77* |
| Placement of LVAD (%) | 2 [0, 5] | 4 | 17/714 | 72* |
| Heart transplantation (%) | 2 [1, 3] | 4 | 24/981 | 0 |
| Complication | ||||
| Angina (%) | 5 [2, 9] | 3 | 5/108 | 0 |
| Myocardial infarction (%) | 4 [2, 6] | 7 | 27/840 | 22 |
| Low cardiac output (%) | 13 [9, 17] | 13 | 119/862 | 80* |
| Atrial fibrillation (%) | 23 [17, 29] | 9 | 294/1,170 | 81* |
| Ventricular tachycardia (%) | 6 [2, 9] | 6 | 39/738 | 76* |
| Bleeding (%) | 3 [1, 5] | 4 | 13/325 | 10 |
| Respiratory failure (%) | 9 [5, 13] | 6 | 49/506 | 55* |
| Cerebrovascular accident (%) | 1 [1, 3] | 19 | 48/2,539 | 19 |
| Infection, unspecified (%) | 11 [5, 18] | 5 | 64/498 | 81* |
| Wound Infection (%) | 3 [0, 7] | 3 | 7/168 | 64 |
| Pneumonia (%) | 6 [3, 11] | 4 | 18/329 | 0 |
| Mediastinitis (%) | 1 [0, 3] | 5 | 5/283 | 0 |
| Sepsis (%) | 3 [1, 6] | 3 | 9/228 | 0 |
| Renal insufficiency (%) | 5 [3, 7] | 19 | 140/1,888 | 75* |
| Dialysis (%) | 3 [1, 6] | 4 | 15/747 | 49 |
| Cause of death | ||||
| Arrhythmia (%) | 3 [1, 4] | 14 | 48/1,118 | 62* |
| Heart failure (%) | 7 [5, 10] | 15 | 108/1,453 | 49* |
| Cerebrovascular (%) | 2 [2, 4] | 12 | 25/1,328 | 0 |
| Multi-organ failure (%) | 2 [1, 3] | 6 | 11/407 | 0 |
| Pneumonia (%) | 2 [0, 4] | 7 | 11/300 | 0 |
| Cancer (%) | 1 [1, 2] | 12 | 28/1,111 | 6 |
| Sepsis (%) | 3 [1, 5] | 5 | 7/194 | 0 |
| Sudden death (%) | 3 [2, 4] | 6 | 25/755 | 0* |
| Unknown (%) | 3 [1, 7] | 5 | 12/569 | 43 |
*, heterogeneity P<0.05 (significant data heterogeneity present). IABP, intra-aortic balloon pump; ICU, intensive care unit.
Discussion
Utilization of SVR has been greatly impacted by the STICH trial. Critics have pointed out flaws in its methodology and interpretation of results (2,10,11), while others have argued against broad application of SVR in ischemic cardiomyopathy (12). This has led to the notion that SVR should be used cautiously in carefully selected patients, which is best reflected in the European Association for Cardio–Thoracic Surgery (EACTS) and European Society of Cardiology (ESC) guidelines on myocardial revascularization in chronic heart failure. Considering SVR with CABG in patients with coronary artery disease, a scarred ventricle and LVESVI >60 mL/m2 is a class IIb recommendation in these guidelines (13).
Of note, the STICH trial was not included in our systematic review/meta-analysis to avoid overlap and double entry of data from its participating institutions. It is worth mentioning that the STICH trial included 501 patients who underwent CABG + SVR (8), while our analysis is based on pooled data from 7,685 patients who all underwent SVR with 92% undergoing concomitant CABG.
The main criticism of STICH is related to patient selection/inclusion and procedural issues. An average reduction of 19% in LVESVI was reported in the CABG + SVR group (8), which is lower than the benchmark of at least a 30% reduction usually achieved by SVR (11). This could have affected the results because postoperative LVESVI is associated with survival. Patients with a postoperative LVESVI greater than 60 mL/m2 have poorer survival than those with an LVESVI <60 mL/m2 (14). Our analysis shows a reduction in LVESVI from 83.9 mL/m2 preoperatively to 46.8 mL/m2 postoperatively—a decrease of 44% and below the 60 mL/m2 threshold for survival benefit. Our results are comparable to the 30% to 50% pre- to post-operative reduction reported in the literature (10,11). This decrease in LVESVI, together with a reduction in the radius of the ventricle, decreases myocardial wall stress according to Laplace’s law. This is a potent means of inducing reverse remodeling (14,15).
Our results show an increase of mean LVEF from 30.0% preoperatively to 40.9% postoperatively. This is comparable to a series reported by Dor et al. where LVEF increased from 26% to 44% one year after SVR (10). In contrast, LVEF of STICH patients undergoing CABG plus SVR increased from 21% to 27% only (8). Variation in technique could account for this difference because more than half of these patients had an LVESVI >60 mL/m2 after surgery (8). After SVR, a mean increase of 10 to 15 points in LVEF has been previously reported (16). This improvement in LVEF is due to the decrease in wall tension and improved contractility resulting from scar removal and restoration of the elliptical shape of the left ventricle (17).
One potential drawback previously reported by Dor is the late worsening of pulmonary hypertension after SVR, which was thought to be due to reduced compliance of the left ventricle by surgical reshaping (7,16). In our analysis, the incidence of pulmonary hypertension trended down from 23% (14–37%) preoperatively to 15% (5–40%) postoperatively (P=0.34). Though this data was limited by the small number of articles with such information reported, the improvement is likely due to an improvement in systolic and diastolic function which subsequently reduces pulmonary pressure. As a result, a reduction in congestive heart failure symptoms is expected, which our results support with an improvement in NYHA functional classification score after SVR. Similar results are also reported by Patel et al. (18).
In our analysis, 21% had concomitant mitral valve repair and 1% had mitral valve replacement. The degree of MR significantly decreased pre to post-operatively (1.6 vs. 0.9) (P<0.01). The Reconstructive Endoventricular Surgery, returning Torsion Original Radius Elliptical Shape to the LV (RESTORE) group has reported similar outcomes, with 23% of their population undergoing mitral valve intervention (22% repair, 1% replacement) (19).
We report an operative mortality of 4% in this meta-analysis. Survival at 1, 5, 10 and 15 years was 92%, 78%, 58%, and 46% respectively. In a recent series, Dor described a 5-year survival of 88% and 79% survival at 100 months post-SVR (10). Isomura et al. from the RESTORE group reported an 8-year survival of 82.4% in patients with postoperative LVESVI <90 mL/m2 while patients with postoperative LVESVI >90 mL/m2 had 0% survival at eight years (19). In the present review, eight-year survival was 68% with an LVESVI of 46.8 mL/m2 postoperatively. Our lower reported survival at eight years is likely due to the inclusion of patients with postoperative LVESVI >90 mL/m2 in included studies. Nonetheless, our results support several other studies, mentioned previously, suggesting the survival benefit of SVR.
Limitations
A large number of retrospective studies are included in the analysis. Due to their risk of bias, much of the data is considered moderate quality in our GRADE assessment. However, this represents the largest dataset of SVR patients to have been analyzed. We were unable to complete a quantitative comparison for CABG vs. CABG + SVR since most studies did not have comparative arms for statistical analysis; therefore, we were unable to report survival benefit. Additional future analysis exploring mitral valve intervention in SVR would be beneficial. We also acknowledge the differences in patient selection and surgical technique of SVR, however it was a fundamental limitation that cannot be addressed when working with pooled data.
Conclusions
Surgical ventricular reconstruction reduces left ventricular volume and improves systolic function in patients with ischemic cardiomyopathy. Our results suggest long term survival comparable to existing reports and good symptomatic improvement following intervention. Further research is still needed to explore the optimal indications of SVR and to identify the group of patients who benefit the most from it.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr Cardiol Rep 2017;19:71. [Crossref] [PubMed]
- Gaudino M, Castelvecchio S, Rahouma M, et al. Results of surgical ventricular reconstruction in a specialized center and in comparison to the STICH trial: Rationale and study protocol for a patient-level pooled analysis. J Card Surg 2021;36:689-92. [Crossref] [PubMed]
- Tennant R, Wiggers C. The effect of coronary occlusion on myocardial contraction. Am J Physiol-Leg Content 1935;112:351-61. [Crossref]
- Cooley DA. Ventricular aneurysm after myocardial infarction: surgical excision with use of temporary cardiopulmonary bypass. Ann Thorac Surg 1988;46:589. [Crossref] [PubMed]
- Dor V, Saab M, Coste P, et al. Left ventricular aneurysm: a new surgical approach. Thorac Cardiovasc Surg 1989;37:11-9. [Crossref] [PubMed]
- Jatene AD. Left ventricular aneurysmectomy. Resection or reconstruction. J Thorac Cardiovasc Surg 1985;89:321-31. [Crossref] [PubMed]
- Dor V, Sabatier M, Di Donato M, et al. Efficacy of endoventricular patch plasty in large postinfarction akinetic scar and severe left ventricular dysfunction: comparison with a series of large dyskinetic scars. J Thorac Cardiovasc Surg 1998;116:50-9. [Crossref] [PubMed]
- Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705-17. [Crossref] [PubMed]
- Calafiore AM, Iaco' AL, Kheirallah H, et al. Outcome of left ventricular surgical remodelling after the STICH trial. Eur J Cardiothorac Surg 2016;50:693-701. [Crossref] [PubMed]
- Dor V, Civaia F, Alexandrescu C, et al. Favorable effects of left ventricular reconstruction in patients excluded from the Surgical Treatments for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2011;141:905-16, 916.e1-4.
- Buckberg GD. Surgical ventricular restoration after flawed STICH trial: results when guidelines followed. Eur J Cardiothorac Surg 2016;50:702-3. [Crossref] [PubMed]
- Toole JM. Surgical ventricular restoration, myocardial viability, and your mother's fine China. J Thorac Cardiovasc Surg 2014;148:2684-5. [Crossref] [PubMed]
- Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. [Crossref] [PubMed]
- Di Donato M, Castelvecchio S, Menicanti L. End-systolic volume following surgical ventricular reconstruction impacts survival in patients with ischaemic dilated cardiomyopathy. Eur J Heart Fail 2010;12:375-81. [Crossref] [PubMed]
- Bruggink AH, van Oosterhout MF, de Jonge N, et al. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J Heart Lung Transplant 2006;25:1091-8. [Crossref] [PubMed]
- Dor V. Left ventricular reconstruction for ischemic cardiomyopathy. J Card Surg 2002;17:180-7. [Crossref] [PubMed]
- Di Donato M, Sabatier M, Toso A, et al. Regional myocardial performance of non-ischaemic zones remote from anterior wall left ventricular aneurysm. Effects of aneurysmectomy. Eur Heart J 1995;16:1285-92. [Crossref] [PubMed]
- Patel ND, Williams JA, Nwakanma LU, et al. Surgical ventricular restoration for advanced congestive heart failure: should pulmonary hypertension be a contraindication? Ann Thorac Surg 2006;82:879-88; discussion 888. [Crossref] [PubMed]
- Isomura T, Hoshino J, Fukada Y, et al. Volume reduction rate by surgical ventricular restoration determines late outcome in ischaemic cardiomyopathy. Eur J Heart Fail 2011;13:423-31. [Crossref] [PubMed]






