Bicuspid aortic valve repair: the 180°-Reimplantation technique
Introduction
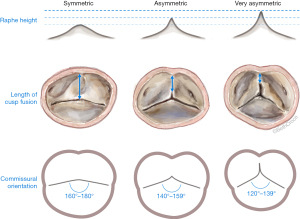
Our understanding of aortic valve pathophysiology has greatly improved with the introduction of the El Khoury classification for aortic regurgitation (AR) early in the new millennium (1). We have learned that aortic valve pathology is not only related to abnormal aortic valve cusps but can also be secondary to dysfunction of the functional aortic annulus (FAA) [aortic root from virtual basal ring (VBR) to sinotubular junction (STJ)], which is sometimes also referred to as the native aortic valve stent. Hence these inter-relationships between aortic valve components are critical for normal valve function and need to be addressed for a durable repair. Our primary surgical strategy for aortic valve regurgitation is valve repair, regardless of valve phenotype. We have learned that bicuspid aortic valves (BAVs) in particular, represent a wide spectrum of valve phenotypes that range from perfectly symmetric valves (180°) to very asymmetric (120°), or almost tricuspid aortic valve like (BAV-Fruste) phenotypes. In a recent analysis of patients with BAVs, a close inter-relationship between commissural orientation, length of cusp fusion and height of the non-functional commissure (the raphe) was uncovered and has greatly improved our understanding of BAV-phenotypes and how we approach these valves surgically (2). Based on these findings, we have then proposed a new repair-oriented surgical classification for BAVs (Figure 1) (3). To facilitate surgical repair, BAV-phenotypes were subcategorized into symmetric-, asymmetric- and very asymmetric-phenotypes, based on commissural orientation (180°–120°). This classification helps to anticipate certain anatomic findings that are related to the valve’s particular phenotype (e.g., raphe height, length of cusp fusion, etc.), and affords the surgeon the ability to come up with a surgical treatment plan in advance.
Over the last three decades we have refined our surgical strategy for BAV-repair based on our learning curves and experiences we have gained over time. In the beginning of our BAV-repair program, we commonly employed subcommissural annuloplasties (Cabrol annuloplasty) that were limited to the commissural areas only. Over time however, our surgical technique evolved to a more customized reimplantation technique: the 180°-Reimplantation technique (El Khoury technique). This technique is a modification of the David 1 procedure, which was originally described for tricuspid aortic valves. Our 180°-Reimplantation technique however, is unique as it creates a symmetric valve and improves the mobility of the fused cusp and increases the aortic valve orifice area that is covered by the non-fused cusp (3). In order to achieve an annuloplasty at the level of the VBR however, a deep aortic root dissection is required (El Khoury maneuver), and the number of annuloplasty sutures is tailored to the respective valve phenotype (selective annuloplasty) (3). We have commonly employed this technique in patients with an aortic root diameter of ≥45 mm. However, in recent years we have also increasingly utilized this technique in patients with an aortic root diameter <45 mm because it provides a superior annuloplasty and stabilization of the entire FAA (from VBR to STJ). It also allows for symmetrization of BAVs, which improves long-term durability and significantly improves freedom from aortic valve reoperation compared to previous repair strategies (4). The following is a detailed description of our signature technique, the 180°-Reimplantation technique.
Operative technique
Preparation
Preoperative assessment includes a baseline electrocardiogram (ECG), chest X-ray, pulmonary function tests, carotid duplex-ultrasound, a coronary angiogram, transthoracic echocardiogram, and a computed tomography (CT)-chest for redo-sternotomies. A transesophageal echocardiogram (TEE) is obtained in the operating room, where baseline parameters are established, such as the size of the aortic annulus, sinus of Valsalva, STJ and ascending aorta. The commissural orientation is measured on short axis view in diastole and determined as the angle measured on the side of the non-fused cusp, between two lines joining the central axis of the aortic root and commissures (3). Intraoperatively, the pattern of cusp fusion, geometric height of the cusps, length of the raphe fusion, and height of the non-functional commissure is evaluated by the surgeon.
Exposition
After median sternotomy and placement of a sternal retractor, systemic heparin is administered, and the patient is centrally cannulated with an aortic cannula [e.g., EOPA arterial cannula (Medtronic, Minneapolis, MN, USA)] in the very distal ascending aorta, and a dual-stage venous cannula, via the right atrial appendage. A root vent is placed for administration of antegrade normothermic blood cardioplegia. Cardiopulmonary bypass is then initiated, and after cross-clamping the first dose of antegrade cardioplegia is administered. This is followed by administration of cardioplegia via direct coronary ostial cannulation every 15–20 minutes. Once the aorta is cross-clamped, a left ventricular vent is also placed through the right superior pulmonary vein.
Operation
Exposure of the aortic root
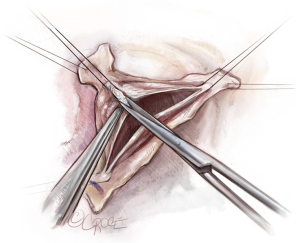
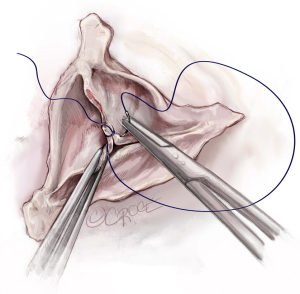
After the heart is arrested, the aorta is incised 1–1.5 cm above the origin of the right coronary artery (RCA). Our routine exposure is as follows: the aorta is incised transversely and almost completely circumferentially. However, the ostium of the left coronary artery (LCA) remains attached to the distal aorta, until LCA reimplantation into the aortic graft is performed later. The anterior wall of the ascending aorta is then suspended to the skin with a 4.0 Prolene suture, and three additional 4.0 Prolene sutures are utilized to suspend each commissure. In this case, two commissures and one raphe (Figure 2).
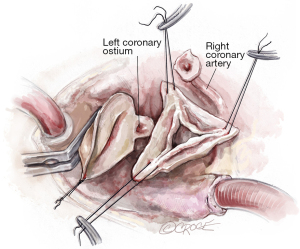
Aortic root dissection
In this case, the origin of the RCA was very high and displaced towards the right/left commissure. The RCA button was therefore created before we performed our usual aortic root dissection. We usually perform the non-coronary sinus dissection first, followed by creation of the RCA button and this is followed by the creation of the LCA button. As stated above, we leave the LCA attached to the distal aorta as it aids with retraction of the aortic root into the surgeon’s field, and it prevents the LCA from twisting before coronary reimplantation (Figure 2).
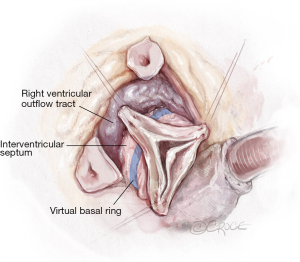
El Khoury maneuver—360° aortic root dissection to the level of the VBR
After the aortic root is prepared with a deep dissection to the level of the VBR at the non-coronary sinus with mobilization of both coronary buttons, the deep root dissection is continued around the right/non-commissure first, paying close attention not to perforate the membranous septum. The area around the membranous septum is the only area where the dissection stops once the fibrous fibers of the membranous septum are identified. All other areas are dissected until the VBR is reached from the outside of the left ventricular outflow tract (LVOT). Hence, the ligament between the pulmonary artery and the LVOT is divided until the right ventricular outflow tract (RVOT) is separated from the LVOT and this is carried forward in a clockwise fashion from the left coronary sinus to the right coronary sinus until the membranous septum is encountered once again. The aortic valve is essentially delivered outside of the heart 360°, by posterior deep dissection behind the fibrous aortic annulus and separation of the RVOT off the LVOT or interventricular septum, thereby exposing the interventricular septum around the left and right coronary sinus of the aortic valve (Figure 3). This assures that the Valsalva-graft later, is seated at the level of the VBR, which is a critical step of the procedure.
Sizing of the Valsalva-graft
In order to size the Valsalva-graft, a straight line is drawn with a marking pen from the nadir of the non-coronary cusp to the nadir of the left-coronary cusp. This line also marks the VBR. Another line is drawn at the tip of non/left-commissure. A flexible ruler is then utilized to measure the distance between the VBR and the tip of the commissure. In this case, the distance was measured at 30 mm, which accordingly translates into a 30 mm Valsalva-graft (Figure 4). In our experience, the sizes generally fall between 28 to 34 mm.
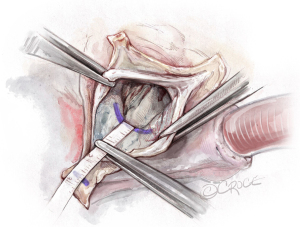
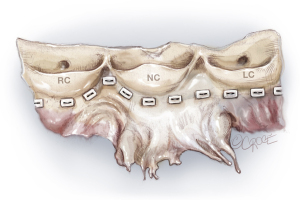
Placement of basal ring horizontal mattress sutures
Pledgetted horizontal mattress sutures (2.0 Ticron) are placed at the level of the VBR 360°. Hence, all sutures are placed at the same level, except around the membranous septum. At the membranous septum we closely follow the line of cusp insertion. Hence, at the non/right-commissure, the sutures before, at, and after the commissure are not placed at the level of the VBR but slightly higher as it follows the insertion of the cusps, to avoid injury to the conduction system (Figures 5,6).
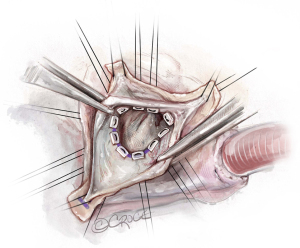
Selective annuloplasty
The degree of asymmetry of the aortic root sinuses varies with the respective BAV-phenotype. The annuloplasty achieved with the reimplantation technique is a combination of the prosthetic graft placed outside of the LVOT and the pledgetted sutures from inside the LVOT, at the level of the VBR. As such, the number of pledgetted sutures co-determines the degree of annuloplasty achieved, which remodels the annulus to acquire a symmetric valve, thereby improving fused cusp mobility. The number of basal ring sutures is individualized based on BAV-phenotype. In this case, because the commissural orientation was around 140° (an asymmetric sinus), we placed one suture at each commissure, five sutures under the fused cusp sinus and three under the non-fused cusp sinus. As the commissures are placed at 180°, it moves the non-fused cusp over to cover a larger orifice area of the aortic valve (Figure 7). This greatly alleviates a transvalvular gradient, in addition to fused cusp modifications later. Another important detail to mention is that selective annuloplasty, relatively increases the free margin length of the fused cusp, and thereby allows for primary closure and completion of the line of fusion of the fused cusp without any tension, simultaneously treating the fused cusp prolapse.
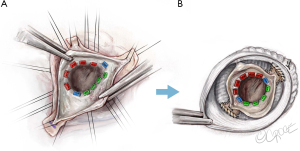
Mobilization and thinning of the raphe
After completing the subvalvular portion of the procedure, the fused cusp is addressed next with mobilization of the raphe off the aortic wall (Figure 8) and thinning of the raphe with sharp Metzenbaum scissors and/or an 11 blade. We do this after the subvalvular sutures are placed to avoid any manipulation of the cusps following cusp repair. This is done to further increase the mobility of the fused cusp. Particular attention has to be paid not to perforate the cusp. The raphe is taken down towards the insertion of the fused cusp. However, the height of the insertion needs to be taken into consideration, as some cusps insert lower than others. This will determine the degree to which the raphe can be detached from the aortic wall. In this case, the raphe was not very fibrotic. However, if needed we would further thin the cusp to improve its mobility.
Free margin thinning
In order to further increase the mobility and pliability of both cusps, the free margins are thinned as needed. This can be done with an 11-blade or sharp scissors (Video 1).
Central cusp plication
The line of cusp fusion is completed with a central plication stitch, which also treats the prolapse of the fused cusp. Depending on the quality of cusp tissues, this is either done with a 5.0 or 6.0 Prolene suture. This is a simple suture taken through the cusp from the aortic side to the ventricular side and then back out to the aortic side and tied down. This is then reinforced with a figure-of-8 stitch (Figure 9). Additional sutures can be used thereafter, if needed.
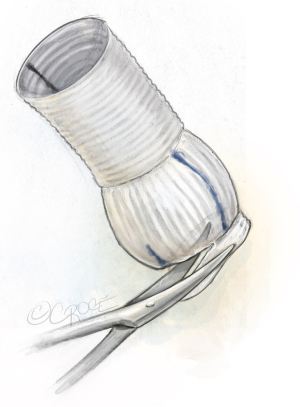
Valsalva-graft preparation
The 30 mm Valsalva-graft is prepared by completely removing the lower skirt of the graft and marking it with a marking-pen at 180° to mark the position of the second commissure, and at 90° to mark the position of raphe (Figure 10).
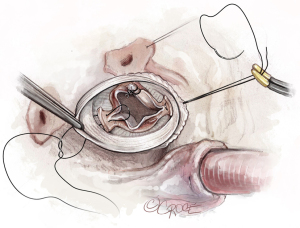
Placement of basal ring sutures in the graft
The basal ring sutures are then placed through the graft starting in the left/right-commissure and going clockwise (Video 1). The commissures are implanted at 180° at the level of the VBR. We place the sutures through the bottom of the Valsalva portion of the graft, except for the non/right-commissure, where the sutures are placed a little higher in order to accommodate the higher placement of the sutures around the membranous septum. The graft is then seated and carefully tied down. The surgical assistant facilitates this step by pulling the graft down with a pair of DeBakey forceps, especially when tying on the interventricular septum.
Reimplantation of commissures at 180°
The two commissures are reimplanted at 180° in the graft and at the level of the neo-STJ, as high as possible with a 4.0 Prolene horizontal mattress suture (Figure 11).
Lowering of the nadir of the fused cusp
The nadir of the fused cusp is then reimplanted in the graft to match the level of the nadir of the unfused cusp. This is also done with a single horizontal mattress suture and is reinforced with the next suture line (Video 1).
Sewing of the graft to the aorta remnant
Starting at the commissures, the graft is sewn to the remnant of the aorta with a running 4.0 Prolene suture. We go full thickness in, through the graft and the aorta, close to the insertion of the cusps; and when we go out, we only take a bite through the graft and around the aortic remnant (Video 1). This suture line, as well as the VBR sutures, both aid in hemostasis.
Dynamic saline test
The graft is then pressurized with cold normal saline utilizing a Tumi syringe, and the valve is assessed thereafter (Figure 12). We check cusp coaptation and assess the valve for a residual prolapse.
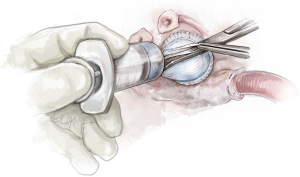
Completion
Following this step, the coronary arteries are reimplanted with a 5.0 Prolene suture and the distal aortic anastomosis is performed with 4.0 Prolene suture (Video 1). The distal anastomosis is performed as far distal as necessary to accommodate a concomitant ascending aorta replacement, as needed. The patient is then weaned off cardiopulmonary bypass. The heart is deaired with a 19-gauge needle through the graft. The valve is then reassessed with TEE. Central trivial regurgitation is acceptable. However, any eccentric jet would be reinterrogated and treated, as it would suggest a residual prolapse.
Comments
Aortic valve repair in general has achieved long-term survival comparable to the general population (4)—results that, to date, have not been achieved with any of the prosthetic aortic valve replacement therapies. We are now starting to see long-term results with BAV repair from different high-volume centers. Although excellent early results are consistently achieved with different surgical techniques, we are beginning to see a divergence of long-term results after 5 years. In a recent report, the Cleveland Clinic shared their intermediate-term experience with BAV reimplantation (5). At 5 years, freedom from aortic valve reoperation was excellent at 94%. However, this declined to 77% at 8 years, as the authors observed progression of AR over time and reoperation in several patients due to severe aortic valve regurgitation.
Our 180°-Reimplantation technique represents the culmination of three decades of aortic valve repair experience at our center. It is a standardized technique that follows certain repair principles for BAV. It entails a selective annuloplasty at the level of the VBR that facilitates symmetrization of the valve and relatively increases the free margin length with overall improvement of fused cusp mobility. We take advantage of the normal more mobile non-fused cusp, in a fashion that allows for this cusp to cover more of the aortic valve orifice area, thereby improving valve opening and alleviating a transvalvular gradient. This is achieved through the aforementioned selective annuloplasty and reimplantation of the commissures at 180°, i.e., a symmetrization of the entire valve complex from VBR to STJ for the entire extent of the FAA. In detail, with our 180°-Reimplantation technique we achieve a symmetric VBR, symmetric sinus depths, symmetric free margin lengths, symmetric cusp geometric heights and a symmetric STJ. We have learned to limit the use of patch material and resuspension of cusp free margins with polytetrafluoroethylene (PTFE) sutures, due to accelerated cusp degeneration over time.
Following these principles, we have achieved consistent and excellent repair results that have previously been reported in a matched analysis that compared our current repair strategy to our earlier repair method (Cabrol annuloplasty) (4). Until 2018, 340 patients had undergone BAV-repair at our center. Of these, 190 were treated with our 180°-Reimplantation technique. Although overall survival was similar between our matched early and late experiences (97% and 94%, respectively), freedom from reoperation and freedom from AR >2+ at 12-year were significantly higher in the reimplantation group, with 91% and 97%, respectively. Hence, not only were we able to achieve excellent early results, but these results remained stable over time. We attribute this to our optimized repair technique which sets our experience apart from other high-volume BAV-repair centers.
We have noticed that one of the key maneuvers that surgeons often shy away from is the 360°-deep aortic root dissection that is required however to perform our 180°-Reimplantation technique correctly and achieve a selective annuloplasty at the level of the VBR. This hesitance grows out of fear of perforation of the RVOT or the interventricular septum. Nonetheless, in our experience this can be done safely, and even if accidental injury should occur, it can be easily repaired through primary suture closure. One helpful maneuver is to leave some volume in the heart following the dissection to check for bleeding, which can then be repaired immediately.
Other surgical strategies to treat BAV-disease are remodeling techniques, with external annuloplasty, or annuloplasty only in normal aortas. Miyahara et al. have recently shared their long-term experience with BAV-repair employing a root remodeling technique in 414 patients and have reported good results, with a 10- and 15-year freedom from reoperation of 88% and 80%, respectively (6).
Nevertheless, we have recently reported our initial clinical experiences with the remodeling technique in patients with connective tissue disorders. Although immediate and intermediate-term outcomes are quite satisfactory, we do observe not only aortic annular dilatation but also recurrence of severe aortic root dilation at 20 years (7). We therefore generally discourage utilizing the remodeling technique in patients with connective tissue disorders, as it appears to be more of a palliative procedure rather than a curative approach in this particular patient population.
In summary, the overall goals for BAV-repair are to increase the mobility of the fused cusp, treat a cusp prolapse and stabilize the repair. We have found that our 180°-Reimplantation technique not only accomplishes all these objectives, but it also achieves excellent long-term durability and freedom from reoperation, as well as overall survival.
Acknowledgments
The authors would like to express their gratitude to Ms. Beth Croce, for rendering and providing the professional surgical illustrations.
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21. [Crossref] [PubMed]
- de Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg 2019; Epub ahead of print. [Crossref] [PubMed]
- Jahanyar J, El Khoury G, de Kerchove L. Commissural geometry and cusp fusion insights to guide bicuspid aortic valve repair. JTCVS Tech 2021;7:83-92. [Crossref] [PubMed]
- de Meester C, Vanovershelde JL, Jahanyar J, et al. Long-term durability of bicuspid aortic valve repair: a comparison of 2 annuloplasty techniques. Eur J Cardiothorac Surg 2021;60:286-94. [Crossref] [PubMed]
- Mokashi SA, Rosinski BF, Desai MY, et al. Aortic root replacement with bicuspid valve reimplantation: Are outcomes and valve durability comparable to those of tricuspid valve reimplantation? J Thorac Cardiovasc Surg 2022;163:51-63.e5. [Crossref] [PubMed]
- Miyahara S, Karliova I, Giebels C, et al. Aortic root remodeling in bicuspid and tricuspid aortic valves-long-term results. Indian J Thorac Cardiovasc Surg 2020;36:81-7. [Crossref] [PubMed]
- Jahanyar J, de Kerchove L, Munoz DE, et al. Twenty-year follow-up after valve-sparing aortic root replacement with the Yacoub or David procedure in Marfan patients. JTCVS Open 2021;7:47-9. [Crossref]






