Twenty-five years’ experience with root remodeling and bicuspid aortic valve repair
Introduction
Bicuspid aortic valve (BAV) anatomy is the most frequent congenital cardiovascular anomaly (1) and is associated with aneurysm of the ascending aorta in half of affected individuals (2,3). This aneurysm involves the root in a significant proportion of individuals (4). The rate of aortic complications rises with increased aortic diameters, prophylactic surgery is offered to prevent the complications (4,5). Replacement will often involve the root, especially if root diameter exceeds 45 mm (5). Surgery may also be necessary to treat relevant aortic regurgitation (AR) (5).
Traditional surgical treatment has consisted of composite replacement of valve and aorta with a mechanical or a biological prosthesis. We have previously proposed root remodeling with concomitant BAV repair for this purpose (6). Root replacement with concomitant valve repair has also shown to have the best durability in patients with severe AR and variable degrees of aortic dilatation (7). It is unclear whether this is due to mechanical stabilization of the root or whether it relates to the fact that remodeling allows for changing commissural orientation into a more symmetric one (7). More than ten years ago, we added a suture annuloplasty to the operation. In a previous report (8), we could not yet find a beneficial effect of the annuloplasty.
The objective of the current analysis is to review our experience encompassing 25 years with this approach. The focus is function of the aortic valve in the second postoperative decade. In addition, we attempt to determine the long-term effect of the suture annuloplasty on late valve function in the setting of BAV and remodeling.
Methods
Between November 1995 and August 2021, 1,213 consecutive patients were treated by aortic root remodeling for AR and aortic root aneurysm. Of these, 31 patients who had undergone aortic valve reimplantation and 710 patients with tricuspid or unicuspid aortic valve morphology were excluded. The remaining 472 individuals had a BAV (Table 1). The outcome of this operation is the subject of the current analysis. This study was approved by the regional ethics committee (Ethikkommission der Ärztekammer des Saarlandes, 202/19).
Table 1
| Characteristics | N=472 |
|---|---|
| Male sex | 429 (90.9) |
| Age (years) | 48±13 |
| Surgical indication | |
| Aortic regurgitation | 317 (67.2) |
| Aortic aneurysm with or without AR | 143 (30.3) |
| Acute aortic dissection | 12 (2.5) |
| Aortic regurgitation grade | |
| 0–I | 19 (4.0) |
| II | 41 (8.7) |
| III | 90 (19.1) |
| IV | 322 (68.2) |
| Cusp fusion | |
| Left/right | 412 (87.3) |
| Right/non | 57 (12.1) |
| Left/non | 3 (0.6) |
| Partial | 115 (24.4) |
| Complete | 357 (75.6) |
| Comorbidities | |
| Coronary artery disease | 25 (5.3) |
| Chronic obstructive lung disease | 8 (1.7) |
| Arterial hypertension | 281 (59.5) |
| Renal insufficiency | 10 (2.1) |
Data are presented as n (%) or mean ± SD. AR, aortic regurgitation; SD, standard deviation.
Surgical technique
The surgical technique has remained more or less constant over time (6,8,9). All operations were performed via a median sternotomy with aortic and right atrial cannulation. In acute dissection, the right axillary artery was used for arterial inflow. Antegrade blood cardioplegia was given directly into the coronary ostia for myocardial protection. A Dacron graft was tailored to accommodate the circumferential orientation of the bicuspid root. Graft size was chosen according to body surface area of the patient, generally 24 mm for patients with a body surface area <2 m2 body surface area and 26 mm for larger patients. In the first 119 patients, the commissures of the nonfused cusp were placed at a 160° orientation. In all subsequent patients, an orientation of approximately 180° was chosen with two symmetric tongues for symmetric and asymmetric BAV (n=309); in analogy to tricuspid valves, three tongues were created for very asymmetric BAV (n=44).
In the initial 58 procedures, valve configuration was estimated visually, using the nonfused cusp as reference for prolapse correction of the fused cusp. Since 2004, the configuration of the nonfused cusp has been measured by determining its effective height (10). If this was less than 9 mm (n=336), prolapse was assumed and central plication sutures were applied for an effective height of 9 to 10 mm. The fused cusp was aligned with the nonfused and its prolapse was generally treated by central plication sutures (n=305). Triangular resection of raphe tissue was performed in the presence of dense fibrosis or calcification (n=129). The fused cusp was reconstructed directly (n=103) or augmented by an autologous pericardial patch tissue (n=26). Autologous patch insertion was also used for closure of perforations (n=3), fenestrations (n=10) or retraction (n=5).
In 201 procedures, no annular stabilization was used. Since 2009, a suture annuloplasty has been added (n=271) using braided polyester (n=50) or expanded polytetrafluoroethylene (n=221). It was tied around a 23-mm (n=79) or 25-mm (n=192) Hegar dilator. Concomitant procedures were performed first, followed by root replacement.
Echocardiography
All patients underwent transesophageal echocardiography intraoperatively (Vivid S70, GE, Boston, Massachusetts, USA). They were studied before discharge, at three months, at one year and biannually after the first two years. Mean and peak systolic gradients were measured (11). AR was analyzed by color Doppler and classified as absent, mild, moderate or severe.
Follow-up
Patients were followed in our outpatient clinic or by the local cardiologists. The first occurrence of AR grade I, II, or III was defined as an event. Follow-up was 92.8% complete and 34 patients were lost to follow-up. Follow-up ranged from one to 275 months (median 61, mean 71±68 months, cumulative 2,585 years).
Statistical analysis
Non-normally distributed continuous variables are presented as median (interquartile range), and the Mann-Whitney U test was used for between-group comparisons. Normally distributed continuous variables are presented as mean ± standard deviation and were compared using the Student’s t-test. Time-dependent data were analyzed using the Kaplan-Meier method. The date of first occurrence of AR was recorded for time-to-event calculation. Data taken from the Kaplan-Meier curve are expressed as mean ± standard error. Categorical data were compared using the chi-square test and are expressed as frequencies (%). Survival and freedom from reintervention were calculated at one, five, ten and 20 years. All statistical tests were two-sided, and P values of <0.05 were considered statistically significant for all analyses.
In logistic regression analysis, a P value less than 0.10 in the univariable analysis was defined for entry into the multivariable analysis. We applied a stepwise procedure for selecting variables based on the Wald criterion of forward induction. All statistical analyses were performed using SPSS 25.0 software (IBM Corp.) (SPSS Inc., Chicago, IL, USA).
Results
The mean patient age was 48±13 years (median 49 years) and ranged from nine to 80 years. The majority of the patients were male (n=429). The most common aortic pathology was aortic aneurysm (n=460); acute aortic dissection was present in 12 individuals.
Relevant AR was present in 322 (68.2%) instances, 60 had only minimal to mild regurgitation. The primary indication for surgery was severe AR (n=317), aortic root aneurysm (n=143) and acute dissection (n=12).
Different fusion types were seen, with right/left fusion being the most frequent (n=412; 87.3%), followed by right/noncoronary fusion (n=57; 12.1%). The diameter of the basal ring varied between 25 to 43 mm (mean 31±4 mm).
Extensive aortic pathology requiring concomitant arch replacement was present in 98 (20.8%) cases. Partial arch replacement was performed in 95 patients, total arch replacement in three patients. Other concomitant cardiac procedures were necessary in 60 instances (Table 2).
Table 2
| Variables | N=472 |
|---|---|
| Annuloplasty | 271 (57.4) |
| Material | |
| Polyester | 50 (10.6) |
| Polytetrafluoroethylene suture | 221 (46.8) |
| Size | |
| 23 mm | 79 (16.7) |
| 25 mm | 192 (40.7) |
| Cusp repair | 452 (95.8) |
| Fused cusp repair | 452 (95.8) |
| Central cusp plication | 305 (64.6) |
| Triangular resection and plication | 103 (21.8) |
| Triangular resection and patch repair | 26 (5.5) |
| Patch repair without triangular resection | 18 (3.8) |
| Nonfused cusp repair | 336 (71.2) |
| Concomitant procedures | |
| Arch replacement | 98 (20.8) |
| Atrial ablation | 18 (3.8) |
| Mitral valve repair | 8 (1.7) |
| Patent foramen ovale closure | 7 (1.5) |
| Tricuspid valve repair | 1 (0.2) |
| Perfusion time (minutes) | 92±25 |
| Myocardial ischemia (minutes) | 68±17 |
Data are presented as n (%) or mean ± SD. SD, standard deviation.
Early outcome
The mean time of extracorporeal circulation was 92±25 minutes and myocardial ischemia was 68±17 minutes (Table 2). In isolated remodeling, mean ischemic time was 65±13 minutes compared to 76±23 minutes with concomitant procedures (P=0.001). Intraoperative conversion after failed repair was not necessary; there was no need for a second period of cross-clamping to correct residual AR.
In-hospital mortality was 0.4% (n=2). One patient died from cerebral hemorrhage due to an intracranial aneurysm, the other from stroke. Eight (1.7%) patients had to be re-explored for hemorrhage and one (0.2%) patient required permanent pacemaker implantation for atrioventricular block following left atrial ablation for correction of persistent atrial fibrillation. At discharge, a competent aortic valve was seen in 427 (90.5%) cases, AR I in 43 (9.1%) cases and AR II in 2 (0.4%) cases (Table 3). The peak systolic gradients ranged from 2 to 40 mmHg with a median of 10 mmHg (mean 10.6±5.8 mmHg).
Table 3
| Variables | N=472 |
|---|---|
| In-hospital mortality | 2 (0.4) |
| Cerebral hemorrhage | 1 (0.2) |
| Stroke | 1 (0.2) |
| Reexploration for bleeding | 8 (1.7) |
| Pacemaker Implantation | 1 (0.2) |
| AR at discharge | |
| No AR | 427 (90.5) |
| AR I | 43 (9.1) |
| AR II | 2 (0.4) |
Data are presented as n (%). AR, aortic regurgitation.
With the addition of an annuloplasty, mean duration of extracorporeal circulation was shorter (89±23 vs. 97±28 min; P=0.001). In addition, myocardial ischemia was shorter with the addition of an annuloplasty (66±14 vs. 71±21 min; P=0.005). The prolonged times were associated with a higher proportion of concomitant procedures (36.2% vs. 27.2%; P=0.043).
Peak systolic gradients were not altered by the addition of an annuloplasty (10.7±5.4 vs. 10.7±6.3 mmHg; P=0.955). No hospital death occurred in conjunction with an annuloplasty. The proportion of competent aortic valves at discharge was higher with (n=260, 94.2%) than without annuloplasty (n=167, 85.2%; P=0.001).
With patch repair of the cusps, the duration of extracorporeal circulation tended to be longer (97±15 vs. 92±26 min; P=0.054). Myocardial ischemia was prolonged (75±13 vs. 68±18 min; P=0.007) despite a trend towards fewer concomitant procedures (18.2% vs. 32.2%). As expected, isolated procedures had significantly longer myocardial ischemia with patch correction (74±13 vs. 64±13 min; P<.001). Systolic gradients were higher with patch repair (12.5±7.0 vs. 10.5±5.7 mmHg; P=0.032). There was no hospital death with patch repair. The proportion of competent aortic valves was similar with (95.5%) and without patch repair (90%; P=0.414).
Late outcome
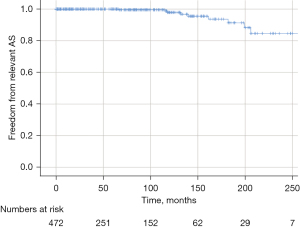
Twenty-two patients died between one and 218 months postoperatively. Overall, nine patients died from non-cardiac and six died from cardiac causes. The cardiac causes were sudden death (n=4) and acute aortic dissection type B (n=2). In seven patients, the cause of death remained unknown. Survival at ten and 20 years was 92.6% and 76.9% (Figure 1).
Reoperation was necessary in 37 cases between one and 259 months postoperatively (Table 4). The main causes of reoperation were AR due to suture dehiscence after cusp repair (n=8, 21.6%), recurrent prolapse due to fenestrations (n=4, 10.8%) or active endocarditis (n=4, 10.8%). Nine patients developed calcific aortic stenosis requiring reoperation.
Table 4
| Variables | N=37 |
|---|---|
| Aortic regurgitation | 24 [65] |
| Suture dehiscence after cusp repair | 4 [11] |
| Suture dehiscence after patch repair | 4 [11] |
| Recurrent prolapse due to fenestrations | 4 [11] |
| Annular dilatation after subcommissural plication | 1 [3] |
| Cusp perforation after healed endocarditis | 3 [8] |
| Cusp restriction | 2 [5] |
| Erosion of suture annuloplasty | 1 [3] |
| Unknown | 5 [14] |
| Active endocarditis | 4 [11] |
| Aortic stenosis | 9 [24] |
Data are presented as n [%].
Overall freedom from reoperation was 90.5% after ten years and 76.6% after 20 years. Freedom from reoperation for aortic stenosis was 97.9% after ten years and 84.6% after 20 years (Figure 2); for AR freedom of reoperation was 92.9% after ten years and 90.9% after 20 years. Recurrent AR grade II occurred in 57 patients. Freedom from AR grade II was 85.1 % at ten years and 55.2% at 20 years.
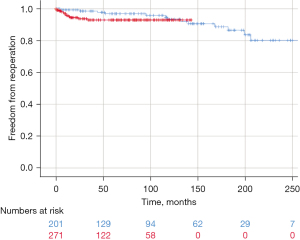
The use of an annuloplasty did not affect ten-year survival (92.4% vs. 91.6%; P=0.394) or ten-year freedom from reoperation (91.7% vs. 91.0%; P=0.438) (Figure 3). In the annuloplasty cohort, only one complication was associated with the annuloplasty (local erosion), leading to reoperation. Ten-year freedom from AR II did not differ between the two groups (84.6% vs. 84.9%; P=0.797).
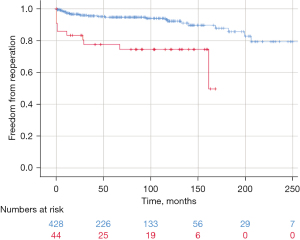
Following cusp repair with a patch, survival after ten years was identical to that without patch (95.4% vs. 92.3%; P=0.542). Freedom from reoperation was higher without patch repair (92.3% vs. 74.5%; P<0.001) (Figure 4). The ten-year durability with patch repair was not influenced by the use of an annuloplasty (78.7% vs. 71.3%; P=0.832). Interestingly, 20-year freedom from reoperation without adding patches to cusp repair was 79.3%.
By logistic regression, age was the only predictor for late death (OR 4.975; P=0.014). Predictors for the need of reoperation were the presence of cusp calcification (OR 2.349; P=0.032) and the use of a pericardial patch for cusp repair (OR 3.684; P=0.003). Pericardial patch repair was also a predictor for recurrent AR II (OR 3.272; P=0.002).
Discussion
The BAV represents a relevant entity due to the valvular complications and the associated aortopathy (1). Because of valve dysfunction with associated aortopathy or pronounced aortic dilatation, a relevant proportion of patients with BAV will require combined treatment of the aorta and aortic valve. The affected individuals are generally younger than those who require treatment for acquired aortic valve disease with implications for life expectancy and lifestyle.
The traditional treatment of combined aortic and valve disease is replacement of valve and aorta, either as a composite or as a separate replacement (12). The disadvantages of these procedures are the need for anticoagulation and valve-related complications (13,14). In addition, excess mortality after valve replacement has been observed in this age group (15). A biologic substitute with its limited durability does not make a realistic choice for younger individuals. With a mean age of 48 years, our current cohort emphasizes these considerations.
Preservation or repair of the regurgitant BAV is an attractive alternative to replacement, particularly due to its low incidence of valve-related complications (16). In fact, repair failure has, by far, been the most frequent valve-related complication (16). There is also new evidence that aortic valve repair may be associated with better survival than replacement (17). On the other hand, repair maintains the bicuspid form and only corrects the deformations that occurred over time. It has been postulated that the bicuspid valve will fail because of its morphology, and the remaining question would be when the valve requires replacement, rather than if (18).
We have pursued the repair approach over the past 26 years and have been able to preserve roughly 90% of non-calcified BAVs over time (19). We learnt that cusp prolapse could be induced by reducing intercommissural distance as part of aortic replacement (9), leading to the introduction of the effective height concept (10). Further experience showed the importance of commissural orientation for valve durability (7). If predictors of failure were avoided, good repair durability could be achieved (20). With the combination of BAV repair and root remodeling, we have observed the best durability (7).
Initially, we tried to maintain the given commissural orientation with an angle of the nonfused commissures of 160°. With the realization that symmetric commissural orientation improves durability and systolic gradients, we have been choosing a symmetric configuration since. We reserve the creation of a tricuspid aortic valve configuration for very asymmetric BAVs (21). In the current cohort, 65.5% of the operations were performed with the symmetric configuration. Given the excellent repair stability, we have applied this approach to moderate root dilatation with sinus diameter exceeding 42 to 45 mm (depending on patient size).
To our knowledge, the current cohort comprises one of the largest series with root replacement and repair of a BAV in unselected patients. The proportion of patients with AR as the indication for surgery was high (67%), and almost all patients required correction of cusp prolapse. Despite the need for concomitant surgery, early morbidity and mortality have continued to be low.
The introduction of an annuloplasty in isolated BAV repair led to marked improvement in repair durability (22). In order to explore whether this concept was also beneficial for root replacement, a suture annuloplasty was added to root remodeling in BAVs (9). Interestingly, the addition of an annuloplasty has not had any effect on ten-year results regarding both survival and freedom from reoperation. The results are thus similar to an earlier study (8). Even though only minimal complications related to an annuloplasty have been observed, the question remains whether its addition has a clinical benefit to the patient.
The other objective of the current investigation was to determine the long-term repair durability in the second decade with BAV repair and root remodeling. With follow-up now reaching 26 years, we have seen stable aortic valve function in the majority of cases. The cumulative incidence of reoperation of 23.4% at 20 years translates into a durability that is better than that of bioprostheses in this age group. Importantly, we introduced the effective height concept only 17 years ago; the patients reaching or exceeding 20 years of follow-up in the current analysis were operated using visual inspection only. With time, the number of patients operated on with this concept will increase and give a more realistic prediction of 20-year results.
Reoperation has mostly been necessary for recurrent regurgitation. This was particularly frequent if cusp repair had included a patch of pericardium. The probability of developing relevant aortic stenosis has been low in the first 15 years after repair. Predictors for the development of stenosis have been calcific plaques in the cusps at the time of the index operation and the implantation of pericardial patches during cusp repair, mostly after excision of a calcified raphe. The current findings are thus similar to previous reports (8,20). Interestingly, the addition of an annuloplasty did not have a positive effect on patch repair durability. This has lowered our threshold for replacement if a patch appears necessary.
Echocardiographically, we have observed a general trend towards more calcium deposition in the cusps in the second decade, even though this has not had an effect on systolic gradients. Despite this, almost all valves with some plaques are still functionally adequate, and further careful follow-up will be necessary to determine the exact role of this observation.
Study limitations
The current analysis is a retrospective single-center study, and we cannot compare the results of remodeling with other variants of valve-preserving root replacement. On the other hand, the clinical results have been good, and myocardial ischemia has been markedly lower than what has been reported for valve reimplantation. Even though we now have an increasing number of patients going into the third postoperative decade, further follow-up will be necessary to determine the true long-term results.
Conclusions
Repair of the BAV combined with root remodeling leads to excellent ten- and 20-year results. The durability is excellent if there is no cusp calcification at the time of surgery and no patch tissue required for cusp repair. Cusp calcification is associated with an increased risk of developing stenosis within 15 years postoperatively. The need for partial cusp replacement with pericardium is the strongest predictor of valve failure and probably should be avoided.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000;83:81-5. [Crossref] [PubMed]
- Nistri S, Sorbo MD, Basso C, et al. Bicuspid aortic valve: abnormal aortic elastic properties. J Heart Valve Dis 2002;11:369-73; discussion 373-4. [PubMed]
- Nkomo VT, Enriquez-Sarano M, Ammash NM, et al. Bicuspid aortic valve associated with aortic dilatation: a community-based study. Arterioscler Thromb Vasc Biol 2003;23:351-6. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. Erratum in: Eur Heart J 2015 Nov 1;36(41):2779. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2022;43:561-632. [Crossref] [PubMed]
- Schäfers HJ, Langer F, Aicher D, et al. Remodeling of the aortic root and reconstruction of the bicuspid aortic valve. Ann Thorac Surg 2000;70:542-6. [Crossref] [PubMed]
- Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85. [Crossref] [PubMed]
- Schneider U, Feldner SK, Hofmann C, et al. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg 2017;153:S65-71. [Crossref] [PubMed]
- Schäfers HJ, Kunihara T, Fries P, et al. Valve-preserving root replacement in bicuspid aortic valves. J Thorac Cardiovasc Surg 2010;140:S36-40; discussion S45-51. [Crossref] [PubMed]
- Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8. [Crossref] [PubMed]
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44. [Crossref] [PubMed]
- Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax 1968;23:338-9. [Crossref] [PubMed]
- Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36:1152-8. [Crossref] [PubMed]
- Oxenham H, Bloomfield P, Wheatley DJ, et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart 2003;89:715-21. [Crossref] [PubMed]
- Kvidal P, Bergström R, Hörte LG, et al. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000;35:747-56. [Crossref] [PubMed]
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32. [Crossref] [PubMed]
- de Meester C, Pasquet A, Gerber BL, et al. Valve repair improves the outcome of surgery for chronic severe aortic regurgitation: a propensity score analysis. J Thorac Cardiovasc Surg 2014;148:1913-20. [Crossref] [PubMed]
- Robicsek F, Thubrikar MJ, Cook JW, et al. The congenitally bicuspid aortic valve: how does it function? Why does it fail? Ann Thorac Surg 2004;77:177-85. [Crossref] [PubMed]
- Miyahara S, Schneider U, Morgenthaler L, et al. (Almost) All Nonstenotic Bicuspid Aortic Valves Should Be Preserved or Repaired. Semin Thorac Cardiovasc Surg 2019;31:656-60. [Crossref] [PubMed]
- Schneider U, Hofmann C, Schöpe J, et al. Long-term Results of Differentiated Anatomic Reconstruction of Bicuspid Aortic Valves. JAMA Cardiol 2020;5:1366-73. [Crossref] [PubMed]
- de Kerchove L, Mastrobuoni S, Froede L, et al. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Schneider U, Hofmann C, Aicher D, et al. Suture Annuloplasty Significantly Improves the Durability of Bicuspid Aortic Valve Repair. Ann Thorac Surg 2017;103:504-10. [Crossref] [PubMed]







