Robotic versus conventional sternotomy mitral valve surgery: a systematic review and meta-analysis
Introduction
Robotic-assisted mitral valve surgery (RMVS) is an evolving technique under the umbrella of minimally invasive valve surgery, so developed out of a need for a less traumatic approach in comparison to the traditional sternotomy. Conventional sternotomy mitral valve surgery (CSMVS), however, remains the gold standard given the unhindered exposure, visual access, and procedural control over instrumentation in the operative site.
Minimally invasive mitral valve surgery, first pioneered by Carpentier and Chitwood in the mid to late-1990s (1), has developed over the subsequent two decades, and allowed cardiac surgeons to circumvent the need for conventional sternotomy at the expense of the degree of access and control these techniques allow. The various techniques developed for performing minimally invasive mitral valve surgery range from partial sternotomy, port-access thoracoscopic, right mini-thoracotomy, and RMVS (2). Although the benefits of minimally invasive surgery over CSMVS are incompletely understood, they are suggested to include better cosmesis, shorter lengths of hospital stay and use of fewer blood products without a significant difference in morbidity and mortality compared to conventional sternotomy (3-5). The downfalls of these more minimally invasive procedures have been consistently related to prolonged operative times which have been attributed to the steep learning curve that minimally invasive mitral valve surgery entails (6).
RMVS, although limited to highly specialized centers, has been undertaken internationally and hopes to boast similar perioperative benefits over traditional approaches; however, evidence for this has been largely limited to single-center studies, and long-term outcome data remains scarce. A 2015 meta-analysis of six retrospective studies performed by Cao et al. showed superior perioperative mortality for RMVS candidates but similar hospitalization and intensive care unit (ICU) stay rates (7). A recent meta-analysis by Takagi et al. analyzed seven propensity-matched studies comparing robotic and conventional sternotomy patients undergoing mitral valve surgery, and conversely found that ICU and hospital length of stay (LOS) were shorter in the robotic group with similar all-cause short-term mortality between the two groups (8). Both of these studies were constrained by the small volume of literature to appraise effectively and systematically. Additionally, the meta-analysis by Takagi et al. included a study which performed a population-based analysis using the National Inpatient Sample database (United States of America) comparing robotic mitral valve repair to non-robotic repair (9). The non-robotic cohort in this study was unable to be further differentiated by surgical approach, and thus included patients who underwent full sternotomy, partial sternotomy and minimally invasive mitral valve repair, therefore introducing bias to the results of that meta-analysis.
Since publication of these two meta-analyses, several single-center analyses have been performed comparing the surgical outcomes of RMVS and CSMVS. The purpose of this study is to perform a comprehensive and rigorous meta-analysis comparing the short-term outcomes of robotic and conventional sternotomy approaches for mitral valve surgery.
Methods
The methods for this meta-analysis adhered to the recommendations and guidelines set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) updated Statement (10).
Literature search strategy
Five electronic databases were used to perform the literature search including Ovid MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CCRCT), Cochrane Database of Systematic Reviews (CDSR), and Database of Abstracts of Review of Effectiveness (DARE). These databases were searched from their inception to 12th March 2022. The search strategy included a combination of keywords and Medical Subject Headings (MeSH) including “Sternotomy” AND “Robotic” OR “Robo*” AND “Mitral valve” AND “Repair” OR “Replacement” OR “Annuloplasty”. Reference lists from previous systematic reviews, meta-analyses and included articles were also reviewed to ensure no additional publications were missed.
Study selection
Study eligibility for inclusion in this systematic review and meta-analysis included those which directly compared RMVS (repair or replacement) to those via conventional full median sternotomy for mitral valve disease. For this meta-analysis, cohorts that were either mixed without reporting separate outcomes, or those comparing partial sternotomy or minimally invasive right anterior thoracotomy mitral valve surgery to the robotically assisted approach were excluded. Studies assessing full cohorts comprised of patients undergoing redo-mitral valve surgery (either by conventional sternotomy or robotic approach) were excluded. If centers reported outcomes of overlapping patient series with either larger cohort size or extended follow-up, the most complete, contemporary series was included for analysis. Included studies were limited to those in English, unless data was easily extractable, and only those involving human subjects. Abstracts, case reports, conference presentations, editorials and reviews were excluded. Title and abstract screening followed by full-text review to determine included studies was performed independently by two reviewers (ML Williams and J Brookes) with any discrepancies discussed until consensus reached.
Outcomes of interest
The primary outcomes of interest were in-hospital/30-day mortality, cerebrovascular accidents (CVA) and re-operation for bleeding. Secondary outcomes of interest included standard operative and post-operative outcomes of interest, for example, renal insufficiency, post-operative atrial fibrillation (POAF), cross clamp time, cardiopulmonary bypass (CPB) time, length of ICU and hospital stay, and post-operative echocardiography results.
Data extraction
For all included studies, two independent reviewers (B Hwang and L Huang) extracted data directly from the reviewed text, tables and/or figures. All extracted data was checked by a senior author (ML Williams) independently, with any discrepancies reviewed, and consensus reached through means of discussion among all three reviewers. Where any indistinct or insufficient data was encountered, attempts were made to clarify these from corresponding authors of the included studies, if required. A priori subgroup analysis was to be performed on matched cohort data separately, therefore, this data was extracted separately to the larger unmatched cohort, where reported.
Statistical analysis
Meta-analysis of means or proportions was performed for categorical and continuous variables, as appropriate, to pool the patient characteristics across the included studies. To facilitate this statistical pooling, the methods described by Wan and colleagues were used to calculate means and standard deviations from the median (with range or interquartile range), where reported (11). This meta-analysis of proportions or means was conducted using Stata (version 17.0, StataCorp, Texas, USA) using a random effects model to account for the different patient populations in the included studies.
Comparative meta-analysis of operative and post-operative variables/outcomes was performed using Review Manager (Version 5.4, Cochrane Collaboration, Software Update, Oxford, United Kingdom). Again, where required for continuous data reported as median values (with range or interquartile range), the mean and standard deviation were estimated using the methods described by Wan et al. (11). A random effects model was used given variation would be present in terms of differing center/surgeon experience, different procedures (repair/replacement), and different operative and management protocols across the included studies. Summary measures were expressed as odds ratios (OR) for dichotomous variables and differences in mean (MD) for continuous data, as appropriate. Data significance and heterogeneity were assessed using the Cochrane Q statistic and the I2 test statistic respectively, with significance set at P value <0.05. Thresholds for heterogeneity significance for I2 values were considered as low, moderate and high heterogeneity at 0–49%, 50–74% and ≥75%, respectively (12). Publication bias was assessed through visual inspection of generated funnel plots.
Study quality appraisal
Study quality of the included studies was assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (13). This quality appraisal tool assesses bias in seven domains, including: bias due to confounding, selection of participants into study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes and selection of the reported result. Using this tool, and assessing the seven domains of bias above, each study can then be classified as either low, moderate, serious or critical risk. Quality appraisal was undertaken independently by two investigators (B Hwang and ML Williams), with any discrepancies rectified through means of discussion until consensus was reached.
Results
The literature search identified a total of 1,459 articles (Figure 1). After exclusion of duplicates and title/abstract screening to remove irrelevant studies, 82 articles were deemed appropriate to undergo full-text review. After full-text review, 68 studies were excluded due to not meeting the inclusion criteria. Therefore, fourteen studies remained which fulfilled the pre-determined inclusion criteria (14-27), including a total of 6,341 patients of which 2,804 underwent RMVS and 3,537 patients underwent CSMVS.
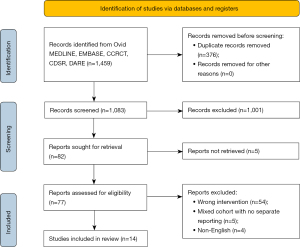
Study characteristics
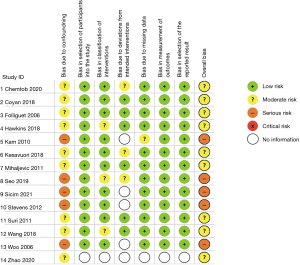
Of the fourteen included studies, thirteen were retrospective comparative studies (15-27) with the fourteenth paper not reporting exactly how the data accrual and analysis were performed (14) (Table 1). Eight studies either included or had separate data for matched patient cohorts, seven of which specifically indicated propensity score matching (PSM) (15,17,19,20,24,25,27), with the eighth study reporting that both cohorts were “matched” retrospectively with no statistical difference in any of the listed patient demographic data in the two cohorts (16). These eight studies included a total of 1,323 matched patients across both the robotic and conventional sternotomy groups. One of the included studies was published in Chinese, however, the published abstract and data listed in tables/figures within the text were in English and therefore included in this meta-analysis due to sufficiently meeting the inclusion criteria (27). The quality of the included studies, which was assessed using the ROBINS-I tool, was deemed to be moderate risk of bias in nine of the included studies (14-17,19,20,24,25,27) and serious risk of bias in the other five included studies (18,21-23,26) (Figure 2).
Table 1
| Primary author | Year | Country | Institution(s) | Study period | Type of study | Robotic (n) | Sternotomy (n) | Follow-up time (months), mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Chemtob | 2020 | USA | Cleveland Clinic, Cleveland, Ohio | 2014–2019 | NR | 605 | 395 | NR |
| Coyan | 2018 | USA | University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania and West Virginia University, Morgantown, West Virgina | 2013–2015 | Retrospective PSM multicenter-center | 91 | 91 | 12 |
| Folliguet | 2006 | France | Institute Mutualiste Montsouris, Paris | 2000–2005 | Retrospective matched single-center | 25 | 25 | 24 |
| Hawkins | 2018 | USA | The Virginia Cardiac Services Quality Initiative (VCSQI) Database | 2011–2016 | Retrospective multi-center* | 372 | 1,352 | NR |
| Kam | 2010 | Australia | Epworth Hospital, Melbourne, Australia | 2005–2008 | Retrospective single-center | 107 | 40 | NR |
| Kesävuori | 2018 | Finland | University Central Hospital, Helsinki | 2011–2015 | Retrospective PSM single-center | 142 | 142 | 35±17 robotic, 64±35 sternotomy |
| Mihaljevic | 2011 | USA | Cleveland Clinic, Cleveland, Ohio, USA | 2006–2009 | Retrospective PSM single-center | 106 | 106 | NR |
| Seo | 2019 | USA | University of California, Los Angeles, California | 2008–2016 | Retrospective single-center | 175 | 259 | NR |
| Sicim | 2021 | Turkey | University of Health Sciences, Gulhane Training and Research Hospital, Ankara | 2014–2020 | Retrospective single-center | 64 | 66 | NR |
| Stevens | 2012 | USA | East Carolina University Hospital, Greenville, North Carolina, USA | 1992–2009 | Retrospective single-center | 447 | 377 | 76.8±54 |
| Suri | 2011 | USA | Mayo Clinic, Rochester, Minnesota | 2007–2010 | Retrospective PSM single-center | 95 | 95 | 1 |
| Wang | 2018 | USA | Duke University Medical Center, Durham, North Carolina-The Society of Thoracic Surgeons (STS) database | 2011–2014 | Retrospective PSM multi-center database | 503 | 503 | 21.36 (11.52–30.96)** |
| Woo | 2006 | USA | University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania | 2002–2005 | Retrospective single-center | 25 | 39 | NR |
| Zhao | 2020 | China | General Hospital of PLA, Beijing | 2002–2014 | Retrospective PSM single-center | 47 | 47 | 6 |
*, study also includes PSM cohorts; **, median and interquartile range. n, number of patients; SD, standard deviation; NR, not report; PSM, propensity score matched.
Patient baseline characteristics
The pooled mean age of patients who underwent RMVS was 63.5 years, slightly younger than those patients which had conventional sternotomy approach, who had a pooled mean age of 64.6 years (Table 2). Both groups had a slight male predominance with 65.5% and 61.8% of patients being male in the RMVS and CSMVS groups, respectively. Just under half of the patients in both groups had a history of hypertension (43.4% and 46.7% for RMVS and CSMVS groups, respectively). The RMVS and CSMVS groups had pooled mean pre-operative left ventricular ejection fraction of 61.5% and 60.5%, respectively. More patients were in New York Heart Association (NYHA) classification class III/IV in the CSMVS group compared to the RMVS group (26.8% and 19.4%, respectively). Myxomatous degenerative mitral valve disease was the main pathology of the mitral valve in both the RMVS and CSMVS groups with 94.6% and 90.5%, respectively. Full break down of underlying mitral valve pathology for surgery for each study can be seen in Table S1. Other pooled patient baseline characteristics can be seen in Table 2. The pooled patient characteristics for the matched patient cohorts can be seen in Table S2.
Table 2
| Variable | Robotic (n=2,804) | Sternotomy (n=3,537) |
|---|---|---|
| Age (years), mean | 63.5 | 64.6 |
| Male, % | 65.5 | 61.8 |
| BMI (kg/m2), mean | 26.0 | 26.5 |
| Hypertension, % | 43.4 | 46.7 |
| Diabetes, % | 4.6 | 7.8 |
| Cerebrovascular disease, % | 3.0 | 4.8 |
| Respiratory disease, % | 4.6 | 8.2 |
| LVEF, mean | 61.5 | 60.5 |
| Cardiac arrhythmia, % | 13.9 | 18.4 |
| PVD, % | 3.4 | 3.7 |
| NYHA III/IV, % | 19.4 | 26.8 |
| Valve pathology—myxomatous degeneration, % | 94.6 | 90.5 |
BMI, body mass index; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease; NYHA, New York Heart Association.
Operative details
Where reported, the operative technique for the RMVS was performed through a varying 2- to 5-centimeter anterolateral mini-thoracotomy and varying number of other access ports. Eleven studies reported the robotic surgical platform used (14-16,18,19,21-24,26,27), all of which used the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, California, USA). Ten of the fourteen included studies reported the robotic cross clamp method. Five reported using solely a transthoracic aortic cross clamp (14-16,22,24), while the other five studies either reported using endoaortic balloon or a transthoracic aortic cross clamp (19,20,23,25,26). Nine studies reported details on cardioplegia delivery with four utilizing antegrade cardioplegia delivery only (14,16,22,24) and five studies reporting using antegrade and/or retrograde cardioplegia (19,20,23,25,26). Most procedures on the mitral valve were a mitral valve repair with 93.8% of patients in the RMVS group and 71.0% of the CSMVS group receiving a mitral valve repair. Concomitant surgical procedures such as left atrial appendage ligation, atrial fibrillation ablation and atrial septal defect closures were reported in seven studies (15,17,19,20,23-25). Eight studies reported events involving intra-operative conversion to sternotomy/thoracotomy (14-17,19-21,24). Forty-three patients across these eight studies converted to larger access incision out of a total 1,766 patients (2.4% conversion rate). Further information on the procedural details, mitral valve repair techniques and concomitant surgical procedures can be found in Table S3.
Mortality
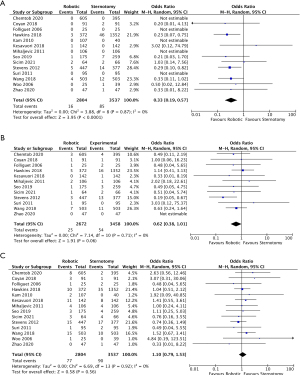
All fourteen studies reported data on all-cause perioperative mortality, however, five studies had no deaths in either cohort. In total, there were 101 deaths (1.6%), of which 16 (0.6%) were in the RMVS group and 85 (2.4%) in the CSMVS. This resulted in a significant difference in all-cause mortality favoring the RMVS group [OR 0.33; 95% confidence interval (CI): 0.19–0.57; P<0.0001, I2=0%] (Figure 3A). When assessing in-hospital all-cause mortality in the matched cohort, a significant difference remained in all-cause mortality favoring the RMVS group (OR 0.35; 95% CI: 0.15–0.80; P=0.01, I2=0%) (Figure S1A). There was no evidence of publication bias on visual inspection of funnel plots.
CVA
CVA events were reported in twelve of the included studies, however, the distinction between transient ischemic attacks (TIA) and permanent strokes were heterogeneously reported so all CVA events were combined. There was a notable difference favoring the RMVS group in regards to rates of CVAs, however, this did not reach statistical significance (OR 0.62; 95% CI: 0.38–1.01; P=0.06, I2=0%) (Figure 3B). When examining rates of CVAs in the matched cohorts (all eight studies), this difference was less noticeable (OR 0.84; 95% CI: 0.43–1.66; P=0.62, I2=0%) (Figure S1B). There was no evidence of publication bias on visual inspection of funnel plots.
Re-operation for bleeding
Rates of re-operation for bleeding were reported in all fourteen studies with 77 patients (2.7%) and 90 patients (2.5%) in the RMVS and CSMVS groups, respectively, requiring re-operation for bleeding. There was no significant difference between the groups in either the total included patients (OR 1.10; 95% CI: 0.79–1.53; P=0.56, I2=0%) (Figure 3C) or matched cohorts (OR 1.28; 95% CI: 0.80–2.03; P=0.30, I2=0%) (Figure S1C). On visual inspection of generated funnel plots, there was no evidence of publication bias.
Secondary outcomes
Sufficient data regarding cross clamp times were reported in twelve of the included studies (14-17,19,21-27). There was a significantly shorter cross clamp time by 28.31 minutes for patients who underwent CSMVS compared with RMVS (95% CI: 19.30–37.32; P<0.00001, I2=97%) (Figure 4A). When assessing matched patient data from seven studies (15-17,19,24,25,27) there remained a statistically significant shortened cross clamp time by 21.54 minutes (95% CI: 12.08–31.00; P<0.00001, I2=93%) (Figure S2A), favoring the CSMVS group. There was no evidence of publication bias on visual inspection via funnel plots.
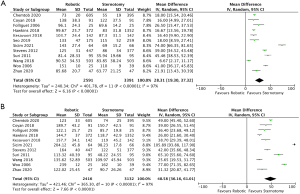
CPB times were reported in eleven of the included studies totaling 5,548 patients (2,416 and 3,132 for RMVS and CSMVS, respectively). The MD between the two groups was statistically significant at 48.58 minutes favoring the CSMVS group (95% CI: 36.16–61.01; P<0.00001, I2=97%) (Figure 4B). Seven studies (15-17,19,24,25,27) reported CPB times for matched patient cohorts and had a shorter MD of 37.81 minutes, favoring the CSMVS group, which was again statistically significant (95% CI: 28.04–47.58; P<0.00001, I2=88%) (Figure S2B). On visual inspection of generated funnel plots, there was no evidence of publication bias.
Data regarding post-operative renal insufficiency was reported in eight studies (14,15,17,21,22,24,25,27). Only 0.7% of RMVS patients suffered from post-operative renal insufficiency, compared to 2.4% of CSMVS patients, resulting in a statistically significant difference between the two groups (OR 0.39; 95% CI: 0.21–0.73; P=0.003, I2=0%) (Figure S3) favoring the RMVS group. There was no evidence of publication bias on visual inspection via funnel plots.
Nine studies reported data on POAF with 25.2% of RMVS patients and 28.3% of CSMVS patients experiencing POAF. When examining the data from the nine studies there was a noticeable difference favoring the RMVS group, however this did not reach statistical significance; importantly, there was a moderate amount of heterogeneity noted (OR 0.81; 95% CI: 0.65–1.01; P=0.06, I2=57%) (Figure S4A). When examining matched cohort data from the five studies reporting POAF data, there was a significant difference favoring the RMVS group (OR 0.73; 95% CI: 0.56–0.95; P=0.02, I2=31%) (Figure S4B) with only a low level of heterogeneity. On visual inspection of generated funnel plots, there was no evidence of publication bias.
ICU LOS data was reported in eleven of the fourteen studies (14-17,19,21,22,24-27). ICU stay was significantly shorter by 26.43 hours (95% CI: −34.31 to −18.54; P<0.00001, I2=96%) (Figure 5A) in the RMVS group compared with the CSMVS group. Matched cohort data regarding ICU LOS was available in seven studies (15-17,19,24,25,27). Again, there was a significantly shortened ICU length of admission in the RMVS group by 21.18 hours (95% CI: −29.20 to −13.16; P<0.00001, I2=93%) (Figure S5A). There was no evidence of publication bias on visual inspection via funnel plots.

Eleven of the included studies reported data on total hospital LOS (14,15,17,19,21-27), six of which contained matched patient data (15,17,19,24,25,27). Hospital LOS was significantly shorter in the RMVS group compared to the CSMVS group by 2.01 days (95% CI: −2.66 to −1.37; P<0.00001, I2=95%) (Figure 5B). When examining matched patient data, a significant difference between the two groups remained, with RMVS group having 1.90 days (95% CI: −2.85 to −0.95; P<0.0001, I2=93%) shorter admission than the CSMVS group (Figure S5B). There was no evidence of publication bias on visual inspection via funnel plots.
Red blood cell transfusion (RBC) data was reported in nine studies (14,16,17,19-21,23-25). There was a significant difference between the two groups (OR 0.44; 95% CI: 0.28–0.70; P=0.0004, I2=89%) (Figure S6A) favoring the RMVS group. This difference remained when assessing only patient-matched cohort data regarding RBC transfusion with lower heterogeneity (OR 0.65; 95% CI: 0.45–0.92; P=0.02, I2=54%) (Figure S6B). There was no evidence of publication bias on visual inspection via funnel plots.
Ventilator time was reported in seven studies with only a total of 994 patients (489 patients RMVS group and 505 patients CSMVS group). The MD between the two groups was 2.6 hours, however, this did not reach statistical significance (95% CI: −6.74 to 1.49; P=0.21, I2=96%) (Figure S7).
Data regarding immediate post-operative or follow-up echocardiography results quantifying mitral regurgitation was reported in ten of the included studies (14-16,18-21,24-26). Due to either the inconsistent or heterogeneously reported data, statistical analysis was not performed. Most patients (where reported) in both the RMVS and CSMVS groups had no/trace/mild mitral regurgitation post-operatively. There was limited follow-up echocardiography data reported in the included studies and where reported high numbers were lost to follow-up. Further details on post-operative echocardiography results can be found in Table S4.
Discussion
Minimally invasive approaches in cardiac surgery are becoming increasingly popular in the attempt to reduce short-term morbidity, and improve cosmesis and return to baseline functionality. However, the safety and efficacy of these minimally invasive approaches, especially in the field of mitral valve surgery, when compared to the gold standard surgical approach through full median sternotomy, remains debated. RMVS, especially robotic mitral valve repair, has more recently had excellent reported outcomes in large volume centers (28,29). However, due to the steep learning curve/operative complexity and higher associated costs, the uptake of robotically assisted approaches in mitral valve surgery has not been widely disseminated (30).
The aim of this meta-analysis was to provide the most updated and comprehensive review of all comparative studies in the existing literature comparing RMVS to CSMVS. Several meta-analyses have previously compared minimally invasive mitral valve surgery (mini-thoracotomy, mini-sternotomy and/or thoracoscopic) to CSMVS (3-5,31-33). However, only two previous meta-analyses have attempted to analyze RMVS and CSMVS (7,8), both of which had their own limitations as mentioned above.
The early all-cause mortality in the present meta-analysis favored the RMVS group in both the total included cohort (0.6% vs. 2.4%; P<0.0001) and matched (0.5% vs. 1.7%; P=0.01) patient cohorts. The meta-analysis of PSM studies by Takagi et al., did not report a statistical difference between RMVS and CSMVS groups for the outcome of all-cause mortality (8). The meta-analysis by Takagi et al., which included seven total PSM studies (six of which are included in the present analysis), included the study by Paul et al., which was the largest PSM cohort included in that study (631 patients in each group) (9). That particular study was excluded from the present analysis as the “non-robotic” cohort included all other types of mitral valve approaches (i.e., full sternotomy, mini-sternotomy and other minimally invasive right thoracotomy approaches) as it was a population-based analysis. The study by Paul et al. reported an OR of 1.21 (95% CI: 0.4–3.62) trending towards the CSMVS group despite not reaching significance, but due to its large patient cohort, likely explains why there was no statistical significance in the all-cause mortality outcome in the meta-analysis by Takagi et al.
Cross clamp and CPB times have consistently been shown to be longer in minimally invasive cardiac surgery procedures when compared to the conventional sternotomy equivalent. Similar to the results seen in the current review, a meta-analysis of 119 studies comparing minimally invasive (not robotic) mitral valve surgery and CSMVS reported that both data from randomized control trials (RCTs) and observational studies showed significantly longer cross clamp and CPB times in the minimally invasive group (4). This meta-analysis reported a mean difference of only 9 minutes for cross clamp time (P<0.05) and 20 minutes for CPB time (P<0.05) when comparing the data from RCTs. The longer cross clamp and CPB times are likely attributable to both the operative complexity of robotic surgery (including docking/undocking and changing instrument arms) and the steep learning curve associated with these procedures. Two of the included studies in the present meta-analysis both reported that total operative, cross clamp and CPB times all reduced in length as operative case numbers increased (19,24). Suri and colleagues compared these times in the first and second half of their included robotic cases for comparison. They reported that the initial mean cross clamp and CPB times of 94.40 and 131.15 minutes, respectively, dropped to 68.67 and 95.85 minutes, respectively, in the second half of their robotic cohort (24). These dramatic improvements in cross clamp and CPB times with more operative experience likely partially explain the high heterogeneity seen in these results in the present meta-analysis, even in the matched patient data analysis.
One of the proposed benefits of minimally invasive cardiac surgery, including robotically-assisted cardiac surgery, is the reduced amount of surgical trauma allowing for a faster recovery to baseline functionality and in theory, shorter ICU and hospital LOS. In the present meta-analysis, RMVS patients had a significantly shorter ICU stay by 26.4 hours (P<0.0001) and a shorter hospital LOS by 2.01 days (P<0.0001); however, both outcomes had significant levels of heterogeneity. The high level of heterogeneity could be due to differing unit protocols for post-operative care and investigations. The shorter ICU and hospital LOS results are consistent with those reported in other meta-analyses comparing minimally invasive mitral valve surgery to CSMVS. A meta-analysis comparing randomized and matched observational studies of minimally invasive to conventional sternotomy for mitral valve repair reported shorter ICU stay by 8.5 hours and hospital LOS by 1.3 days in the minimally invasive group, both results also having high heterogeneity (I2>90%) (5).
Similar to the findings by Takagi et al., this present meta-analysis found that the incidence of RBC transfusion was lower in the RMVS group than the CSMVS group in both the total included cohort (OR 0.44; P=0.0004) and matched cohort (OR 0.65; P=0.02). These results need to be interpreted with caution, as two of the included studies reporting data on RBC transfusion defined transfusion as greater than or equal to two units of RBCs, therefore introducing significant heterogeneity (19,24). However, reduced rates of RBC transfusion have been consistently reported across most meta-analyses comparing minimally invasive surgery to CSMVS (3-5).
There are a number of important limitations to consider when interpreting the results described in this present meta-analysis. Firstly, despite including fourteen studies comparing the safety and efficacy of RMVS to CSMVS, they were all retrospective observational series. Eight studies (15-17,19,20,24,25,27) included matched patient data which does to some degree minimize selection bias, however, as no studies were randomized, there inherently remains the risk of selection bias. Secondly, there was a heterogenous mix of procedures including mitral valve repair and replacement in most studies, with only six studies including cohorts of either purely mitral valve repair or replacement (16,18,21,22,24,27). Thirdly, along with the heterogenous procedures, the included studies also included heterogenous cohorts of mitral valve pathologies, which is an important consideration when interpreting the results. Fourthly, significant heterogeneity was detected in the analyses of cross clamp time, CPB time, RBC transfusion, ventilator time, along with ICU and hospital LOS. This may reflect the limited data, different surgical techniques, and specific unit protocols or operator experience across the included studies. Finally, data regarding post-operative echocardiography results were limited and heterogeneously reported. In future studies, direct comparison of mitral valve repair echocardiography results post-operatively between both surgical approaches with complete, long-term follow-up are required.
Conclusions
Both surgical approaches to mitral valve surgery have an adequate safety and efficacy profile. Current evidence on comparative outcomes of RMVS and CSMVS is limited, with only low-quality studies currently available with moderate-to-serious risk of bias. This present meta-analysis shows that RMVS may result in lower mortality, along with shorter ICU and hospital LOS compared to CSMVS in selected patients. On the contrary however, CSMVS may be associated with significantly shorter cross clamp and CPB times. Further high-quality studies with randomized data are required to verify these results and also necessary to assess differences in mitral valve repair quality and postoperative quality of life differences between the two surgical approaches.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: TSG provides consultation for Edwards Lifesciences, Johnson & Johnson, and Intuitive Surgical. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23. [PubMed]
- Burns DJP, Wierup P, Gillinov M. Minimally Invasive Mitral Surgery: Patient Selection and Technique. Cardiol Clin 2021;39:211-20. [Crossref] [PubMed]
- Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [Crossref] [PubMed]
- Eqbal AJ, Gupta S, Basha A, et al. Minimally invasive mitral valve surgery versus conventional sternotomy mitral valve surgery: A systematic review and meta-analysis of 119 studies. J Card Surg 2022;37:1319-27. [Crossref] [PubMed]
- Sá MPBO, Van den Eynde J, Cavalcanti LRP, et al. Mitral valve repair with minimally invasive approaches vs sternotomy: A meta-analysis of early and late results in randomized and matched observational studies. J Card Surg 2020;35:2307-23. [Crossref] [PubMed]
- Toolan C, Palmer K, Al-Rawi O, et al. Robotic mitral valve surgery: a review and tips for safely negotiating the learning curve. J Thorac Dis 2021;13:1971-81. [Crossref] [PubMed]
- Cao C, Wolfenden H, Liou K, et al. A meta-analysis of robotic vs. conventional mitral valve surgery. Ann Cardiothorac Surg 2015;4:305-14. [PubMed]
- Takagi H, Hari Y, Nakashima K, et al. Meta-analysis of propensity matched studies of robotic versus conventional mitral valve surgery. J Cardiol 2020;75:177-81. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Jalbert J, et al. A population-based analysis of robotic-assisted mitral valve repair. Ann Thorac Surg 2015;99:1546-53. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Chemtob RA, Wierup P, Mick SL, et al. A conservative screening algorithm to determine candidacy for robotic mitral valve surgery. J Thorac Cardiovasc Surg 2022;164:1080-7. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Folliguet T, Vanhuyse F, Constantino X, et al. Mitral valve repair robotic versus sternotomy. Eur J Cardiothorac Surg 2006;29:362-6. [Crossref] [PubMed]
- Hawkins RB, Mehaffey JH, Mullen MG, et al. A propensity matched analysis of robotic, minimally invasive, and conventional mitral valve surgery. Heart 2018;104:1970-5. [Crossref] [PubMed]
- Kam JK, Cooray SD, Kam JK, et al. A cost-analysis study of robotic versus conventional mitral valve repair. Heart Lung Circ 2010;19:413-8. [Crossref] [PubMed]
- Kesävuori R, Raivio P, Jokinen JJ, et al. Early experience with robotic mitral valve repair with intra-aortic occlusion. J Thorac Cardiovasc Surg 2018;155:1463-71. [Crossref] [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Seo YJ, Sanaiha Y, Bailey KL, et al. Outcomes and Resource Utilization in Robotic Mitral Valve Repair: Beyond the Learning Curve. J Surg Res 2019;235:258-63. [Crossref] [PubMed]
- Sicim H, Kadan M, Erol G, et al. Comparison of postoperative outcomes between robotic mitral valve replacement and conventional mitral valve replacement. J Card Surg 2021;36:1411-8. [Crossref] [PubMed]
- Stevens LM, Rodriguez E, Lehr EJ, et al. Impact of timing and surgical approach on outcomes after mitral valve regurgitation operations. Ann Thorac Surg 2012;93:1462-8. [Crossref] [PubMed]
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [Crossref] [PubMed]
- Wang A, Brennan JM, Zhang S, et al. Robotic Mitral Valve Repair in Older Individuals: An Analysis of The Society of Thoracic Surgeons Database. Ann Thorac Surg 2018;106:1388-93. [Crossref] [PubMed]
- Woo YJ, Nacke EA. Robotic minimally invasive mitral valve reconstruction yields less blood product transfusion and shorter length of stay. Surgery 2006;140:263-7. [Crossref] [PubMed]
- Zhao H, Zhang H, Yang M, et al. Comparison of quality of life and long-term outcomes following mitral valve replacement through robotically assisted versus median sternotomy approach. Nan Fang Yi Ke Da Xue Xue Bao 2020;40:1557-63. [PubMed]
- Arghami A, Jahanian S, Daly RC, et al. Robotic Mitral Valve Repair: A Decade of Experience With Echocardiographic Follow-up. Ann Thorac Surg 2021;S0003-4975(21)01919-6. Epub ahead of print. [Crossref] [PubMed]
- Roach A, Trento A, Emerson D, et al. Durable Robotic Mitral Repair of Degenerative Primary Regurgitation With Long-Term Follow-Up. Ann Thorac Surg 2022;114:84-90. [Crossref] [PubMed]
- Benmessaoud C, Kharrazi H, MacDorman KF. Facilitators and barriers to adopting robotic-assisted surgery: contextualizing the unified theory of acceptance and use of technology. PLoS One 2011;6:e16395. [Crossref] [PubMed]
- Cao C, Gupta S, Chandrakumar D, et al. A meta-analysis of minimally invasive versus conventional mitral valve repair for patients with degenerative mitral disease. Ann Cardiothorac Surg 2013;2:693-703. [PubMed]
- Hage A, Hage F, Al-Amodi H, et al. Minimally Invasive Versus Sternotomy for Mitral Surgery in the Elderly: A Systematic Review and Meta-Analysis. Innovations (Phila) 2021;16:310-6. [Crossref] [PubMed]
- Sündermann SH, Sromicki J, Rodriguez Cetina Biefer H, et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:1989-1995.e4. [Crossref] [PubMed]






