Historical evolution of robot-assisted cardiac surgery: a 25-year journey
Introduction
“A robot may not injure a human being.”
“A robot must obey the orders given to it by human beings.”
—Isaac Asimov’s First and Second Law of Robots
The lore of automated devices to do tasks for human beings or fight battles has existed from ancient times. In his book Mechanike syntaxis (Compendium of Mechanics), Philon of Byzantium (220 BC), also known as Philo Mechanicus, described a robot servant that could mix wine from an amphora in one hand and water in a cup that was placed in the other hand. Later, Heron of Alexandria (10–70 AD) developed a steam driven (aeolipile) automated “programable” device that with pulleys and ropes could open doors and deliver various automated devices onto a theater stage. Leonardo daVinci (1495) fabricated a knight robot in which cogwheels, helical screws, pulleys, and ropes could activate human-like arms to emulate human motions (Figure 1). In his 1564 book, Instrumenta Chyrurgiae et Icones Anathomicae, Ambrose Pare illustrated what was probably the first mechanical hand prosthesis. Activated by springs, miniature cogwheels, and catches, the fingers would move up and down.

Modern innovators are developing unique robots that can emulate partially the motion range of the human hand. These “hand-like” robots are ingenious with some using artificial intelligence directed machine learning to control either mechanical actuators or piezoelectric pneumatic micro pumps. These devices can mimic hand and finger actions reasonably well. However, to reproduce the necessary freedom of motion provided by the versatile combined human anatomy has been impossible.
The term “robot” was derived from the Czech word robota, which means forced labor to do meticulous work. In 1921 Karel Čapek, who was nominated for the Nobel Prize in literature seven times, wrote (R.U.R) Rossum’s Universal Robots where in his melodrama industrialists planned to usurp human productivity by replacing people with working robots (1). These were not like robots as we know them but human-like “biological” machines. In the play Čapek wrote:
“Young Rossum invented a worker with the minimum number of requirements. He had to simplify him. He rejected everything that did not contribute directly to the progress of work! - everything that makes man more expensive. In fact, he rejected man and made the Robot. … the Robots are not people. Mechanically they are more perfect than we are, … they have an enormously developed intelligence, but they have no soul.”
Now, roll the calendar forward almost 100 years—robots have become major “workers” in many industries. They have been shown to perform repetitive tasks with accuracy and efficiency. The evolution and application of robot-assisted surgery has been much slower. Perhaps this has resulted from the contention that any mechanical device, even combined with assisted vision, could never reproduce the abilities of a skilled surgeon’s hands. No doubt when operating through large surgical incisions, the conjoint motion of the human shoulder, arm, elbow, wrist, and fingers easily can outperform any multi-articulated robot. However, to perform miniaturized surgical tasks through tiny entrance ports, the needed ergonomics provided by multifunctional human anatomy become impossible. For example, when operating with long shafted instruments, even with endoscopic vision, small incisions become a pivot-axis, which reverses eye-hand motions that limit access to many areas of the operative field. The surgeon must think in the “verso” to perform each task.
Early minimally invasive cardiac surgery (MICS)
“The surgical incision provides no therapeutic benefit.”
—Catherine Mohr, MD, Intuitive Surgical
The discussion of evolving robot-assisted cardiac surgery would be incomplete without reviewing important premonitory advances in MICS. As stated above, the surgical incision has only one purpose—to provide access to the surgical site. Although large incisions provide wide access, they often prevent rapid patient recovery and have completed their service after the operation. The therapeutic parts of most operations generally relate either to extirpation, reconstruction, or implantation. Because of surgical traditions, we older surgeons were taught to have the widest exposure possible for patient safety and the best clinical results. However, in the modern era there has been a stepwise evolution toward the least invasive operations with the same excellent results. To this end, non-robotic MICS became the springboard for the development and acceptance of robot-assisted operations.
Historically, in 1967, Kolosov performed the first internal thoracic to coronary artery bypass on a beating heart through a thoracotomy (2). In 1975, Ankeney, then at Case Western Reserve University, wrote an editorial supporting “off pump” coronary surgery, which he had adopted as early as 1967 (3). He then reported the twenty-four-year follow-up of 241 patients that he had operated upon “off pump” through a sternotomy (4). Between 1981 and 1996, Buffolo in Brazil performed 1,274 “off pump” coronary operations through a sternotomy with a 2.5% mortality (5). He claimed that a major benefit of this approach was both decrease in complications and reduced economic costs. In a subsequent commentary, Ullyot rebuffed Buffalo’s series of “off pump” operations, cautioning surgeons not to abandon the gold standard of a sternotomy-based “on pump” surgery just to save cost (6). In Argentina, Benetti first developed the off pump “MIDCAB” or “keyhole” coronary operation (1994), which was done either under direct vision or with endoscopic visualization through a mini thoracotomy (7). He also performed the first “OPCAB” where, through a mini thoracotomy, the internal thoracic artery (ITA) was mobilized endoscopically and additionally anastomosed to the left anterior descending coronary artery (LAD) (8). Thereafter, Benetti and Ferrari, an entrepreneur, founded Cardiothoracic Systems Inc. (Cupertino, California) to develop and manufacture new minimally invasive retractors and coronary stabilizers. This company later was acquired by Guidant, Inc. By 1995 Califiore had performed 155 “off pump” mini-thoracotomy coronary operations (9). Nataf reported his series of thirty videoscopic coronary operations, with one graft occlusion and two stenoses by angiography (10). In his conclusions he stated,
“The efficiency of this minimally invasive approach should be prospectively compared with similar revascularization with PTCA or surgical approaches using sternotomy with or without CPB.”
Mack and associates performed early video-assisted coronary operations through a mini-thoracotomy using a head mounted display called Vista (11). Beginning in 1994, Borst, Jansen, and Grunderman began to develop in Utrecht, Netherlands the “Octopus” beating heart suction stabilizer, which remains as a major step forward in “off pump” coronary surgery (12). In many of these early minimally invasive coronary operations, the left ITA was anastomosed to the anterior descending coronary artery. However, today multivessel “off pump” revascularization has become one standard of care. Each of these forgoing pioneers and other innovators initiated the beginning of both video-assisted minimally invasive and “off-pump” coronary surgery, which expanded worldwide in the next near thirty years (13).
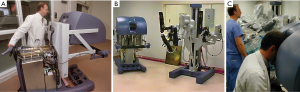
As a research fellow, Dr. William Peters developed the first endoballoon prototype in the Stanford research laboratory and then filed for a patent in 1993, receiving it in 1995 (Figure 2A,2B) Then innovative surgeons and engineers further developed the endoballoon aortic occlusion device and the Heartport Port Access (PA) technique (14,15). In addition, they invented the first long shafted instruments that were designed just for MICS. The first PA coronary and mitral replacement operations were done in 1995 at Stanford University and in October of 1996 in Kuala Lumpur, Malaysia, respectively (16) (Figure 3A-3C). Reportedly, both Drs. Cosgrove of the Cleveland Clinic and Cohn of the Brigham and Women’s Hospital, observed several early PA operations at Stanford, and this prompted them to begin their minimally invasive valve programs (17,18).
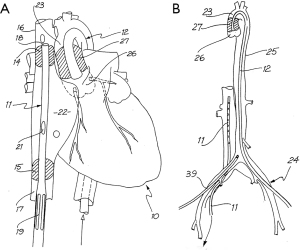
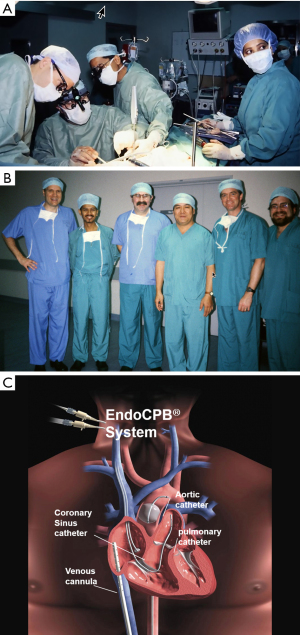
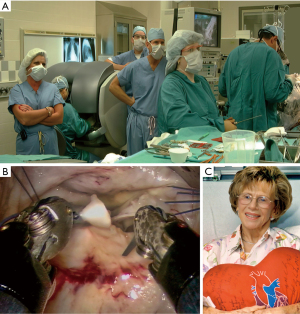
On February 26, 1996, Carpentier performed the first video-assisted mitral valve repair, which was done through a mini-thoracotomy using ventricular fibrillation (19). In early February of 1996, Benetti replaced a mitral valve through a small thoracotomy using a 3-dimensional camera and the Vista head-mounted display (20,21) (Figure 4). Several weeks later at the First International Live Teleconference on Less Invasive Coronary Bypass Surgery (Oxford, England), hehighlighted use of the Vista device for coronary surgery (22). On May 26, 1996, our group at East Carolina University performed the first mitral valve replacement in North America through a mini thoracotomy using a two-dimensional (2D) endoscope and our newly developed trans-thoracic aortic clamp along with retrograde cardioplegia (23) (Figure 5A-5C). At the 1997 meeting of the American Association for Thoracic Surgery, we presented our first experience of thirty-one video-assisted mitral operations with one death and no major complications (24). At the same meeting, Mohr reported the Leipzig Heart Center experience using PA technology, a 4-cm incision, and a two-dimensional endoscope. Of fifty-one mitral operations, there was a 9.8% mortality and a significant number of serious complications including, aortic dissections and untoward neurologic events. Mohr’s bravery was championed by other surgeons for presenting this series, which was to place a temporary pall over the PA aortic endo-occlusion method (25). Baldwin then wrote a critical editorial and suggested that a randomized trial be done to compare the minimally invasive PA method with conventional sternotomy operations (26). However, over the years both the endoaortic occlusion device and implementation improved markedly. Device safety became evident by large successful the early clinical series of Vanermen, Reichenspurner, Mohr, Hargrove, Galloway, and Glower, among others (27-32). A recent comprehensive study by Balkhy et al. showed comparable safety and efficacy to trans-thoracic aortic clamping (33). Some of the surgeons who were responsible for many of these early pioneering contributions to mitral valve MICS include, but are not limited, to Carpentier, Chitwood, Colvin, Falk, Galloway, Grossi, Hargrove, Loulmet, Mohr, Reichenspurner, Vanermen, and Wimmer-Greinecker. Using both the PA and trans-thoracic aortic clamping methods, these individuals established MICS as one standard of care, which became the springboard to develop robot-assisted cardiac surgery.
Early development of cardiac surgical robots
“From Skepticism to Standard of Care.”
—Dr. Richard Satava (Colonel US Army)
In the book entitled Surgical Robotics: Systems Applications and Visions, the authors comprehensively detail the exciting early development and application of surgical robots (34). When Scott Fisher worked at the NASA Ames Laboratory (1985–1990), he led the Virtual Environment Workstation Project that developed the first a head mounted three-dimensional image display, which provided what he first called it “telepresence” (35). The first attempt to develop “tele-surgery” began in 1987 at the Stanford Research Institute (SRI) by Philip S. Green, a biomedical engineer and Joseph Rosen, a plastic surgeon (36). The system, which Green called the “telepresence surgical system”, was somewhat similar to contemporary master-slave robotic surgical systems in that the operator worked from a remote workstation to manipulate end-effector instruments (Figure 6A,6B). This device provided 3-D visualization and had detachable instrument tips, which were limited to four degrees of motion freedom but provided the operator some forced feed-back. The SRI received funding from the NIH to develop a prototype robotic surgical system. At that time Dr. Richard Satava (Colonel US Army), a program manager for Defense Advanced Research Projects Agency or DARPA, began to work with Green at the SRI to develop a military based tele-presence surgical system that would allow surgeons to operate remotely on injured soldiers at battlefield locations (35,37). This “tele-surgery” project was funded by DARPA.
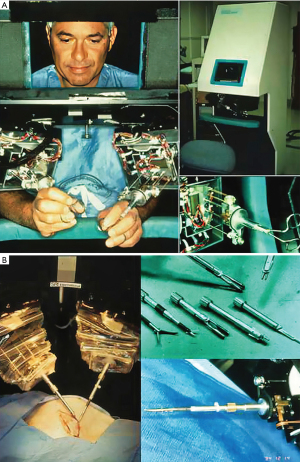
Dr. Satava has stated: “…the robot was not a machine but rather an information system, which could integrate all the capabilities (tele-communications, remote imaging, computer enhanced manipulation, image acquisition, and guidance) needed to support remote surgery.”
In June of 1993 first tele-surgical remote procedure was performed on an ex-vivo porcine intestine, which was located at a distance in a “mock battlefield” military vehicle (34,35). Then in 1994 Dr. John Bowersox, a vascular surgeon, performed an experimental arterial anastomosis and patch aortoplasty (38). Between 1995 and 1998, Green patented this device as Green Telepresence Surgery System.
Computer motion—AESOP and ZEUS
The voice-controlled robotic camera positioning arm, called the AESOP 1000, (Automatic Endoscopic System for Optimal Positioning) was developed by Computer Motion Inc, which was founded in 1990 by Yulun Wang PhD. In 1993 this was the first US Food and Drug Administration (FDA) approved surgical robot. The second-generation, AESOP 2000, enabled surgeons to have 23 precise tremor-free voice-activated camera positions. By 1998, the new AESOP 3000 added more degrees of camera motion. This device provided more camera stability and less lens cleaning, which translated into reduced cardiopulmonary bypass and cross-clamp times. This technology enabled use of even smaller incisions with better valve and subvalvar visualization. Guided by AESOP 3000 endoscopic visualization, we performed many minimally invasive mitral operations without requiring a first assistant (Figure 7). In fact Mohr coined term “solo surgery” after having performed 137 mitral operations using this device (39).
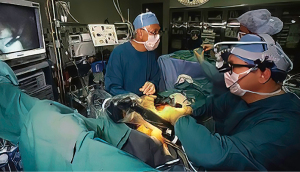
In 1996, Computer Motion, Inc. launched the ZEUS surgical robot, which when commercialized had a lower selling price than the competitive daVinci system (Figure 8). It was comprised of operating table mounted instrument arms with end-effectors that when activated had 4 degrees of motion freedom. Surgeons operated from a separate console using hand activators. Visualization was through a large monitor with the operator wearing 3-dimensional eyeglasses. On September 15, 1999, in Munich, Germany, Reichenspurner, Damiano, and Mack performed the first two ZEUS coronary bypass operations (40). In Canada, Boyd and Rayman reported their first series of beating heart totally endoscopic coronary bypass (TECAB) operations that were first done on September 18, 1999 (41). In early May of 2000, Loulmet and Grossi performed a ring annuloplasty of using the Zeus system (42). On September 7, 2001, the first and only trans-continental robot-assisted operation, a cholecystectomy, was performed between New York City and Strasbourg, France by Marescaux, thus proving the possibility of safe long distance telesurgery (43,44). Later, this operation was given the soubriquet of “the Lindberg Operation”, referring to Charles Lindberg’s historic trans-Atlantic flight. Because of patent infringement lawsuits by Computer Motion, Inc., and counter lawsuits by Intuitive Surgical, Inc., in 2003 the two companies merged into the later company. Soon after the ZEUS robot was withdrawn from commercial sales.
Intuitive—daVinci system
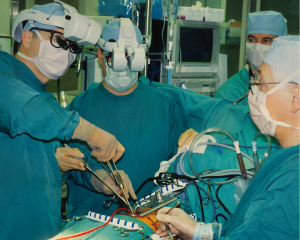
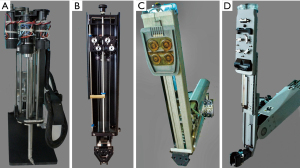
In 1994 Dr. Fred Moll, an innovative surgeon and entrepreneur, became interested in the SRI telepresence system. He left his position at Guidant, Inc. and with Dr. John Freund and Rob Younge, an electrical engineer, developed the business plan to form the Intuitive Surgical company, which was founded in 1995. They then raised funds from venture capitalists and several other sources to purchase the intellectual property from SRI, IBM, and Massachusetts Institute of Technology (MIT) (34,35). Based on the patents derived from the Green Telepresence System, over the next three years the Intuitive Surgical engineers developed several iterative prototypes (Figure 9A-9D). Lenny (1996) was the first robotic arm to have seven degrees of motion freedom. In 1997, using the next prototype, called Mona, Shennib, Mack and Moll anastomosed in ex-vivo pig hearts excised circumflex coronary arteries to in-situ left anterior descending coronaries (45). That year, I was fortunate to perform similar coronary anastomoses with the same device at the Intuitive Surgical laboratory then in Mountain View, California (Figure 10A,10B). Albeit, that Mona needed significant refinements in ergonomics and visualization, this was, as Archimedes once shouted, a “eureka* moment for me when. I had just realized that this disruptive technology would eventually become a major advancement for many surgical procedures and in specific minimally invasive cardiothoracic operations. *Eureka from the Greek heureka meaning, “I have found it”.
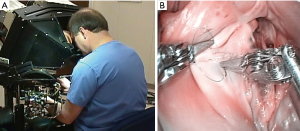
Operating with the Mona prototype at St. Blasius Hospital in Belgium, Himpens and Cadier performed the first robot-assisted cholecystectomy on March 3, 1997 (46). On May 7,1998 at Broussais Hospital in Paris, Carpentier and Loulmet completed successfully the first robotic mitral repair with this device (Figure 11) (47). Beginning sixteen days later, Mohr and Falk did five mitral valve repairs successfully at the Leipzig Heart Center with the same prototype (48) (Figure 12A-12C). Then, on May 25,1998 the same surgeons performed the first two robot-assisted ITA-LAD anastomoses using the Heartport PA/cardioplegia method (49). Thereafter, in June of 1998, Loulmet and Carpentier completed successfully two ITA to LAD robot-assisted operations on the arrested heart (50). Between 1998 and 1999, Falk and Mohr the performed twenty-two similar daVinci ITA to LAD coronary operations on the arrested heart and first coined the term “TECAB” for the totally endoscopic coronary artery bypass operation (51). They also showed a progressive decrease in ITA mobilization times with increasing operative experience. On March 27, 2000, in Dresden, Germany, Kappert et al. effected the first daVinci beating heart TECAB (52). These pioneering surgeons, and several others confirmed that the developing daVinci system would be safe and efficacious for both mitral valve and coronary artery operations. Thus, they catapulted into existence the new era of robot-assisted cardiac surgery.

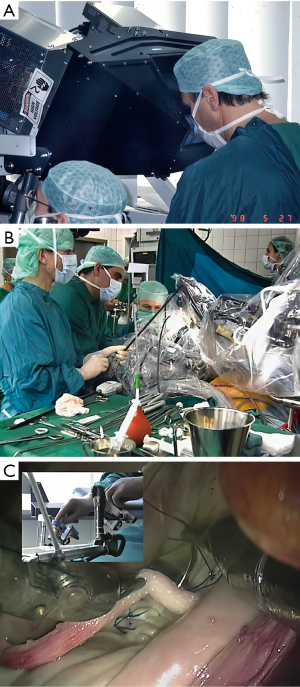
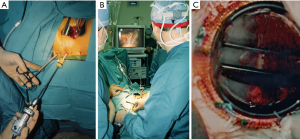
The first commercial daVinci was purchased in 1999 by the Leipzig Heart Center. That year, our group at East Carolina University acquired the first commercial daVinci robot in the United States, albeit not yet FDA approved for intra-cardiac use (Figure 13A-13C). Wolf and Michler were the first (September 12, 1999) to harvest an ITA in the United States with the daVinci system. As an FDA institutional device exemption (IDE) clinical trial, they performed twenty-four ITA mobilizations in patients over the next six months (53). In each case a hand sewn anastomosis was made to the left anterior descending artery. By angiography they compared anastomoses done using daVinci system harvested ITAs (N=15) to those mobilized by traditional sternotomy methods (N=10). Excellent ITA-LAD patency in both groups led to FDA approval for robot-assisted graft mobilization. After almost a year of experimentation, planning, and practicing in the research laboratory using the daVinci system, we began an FDA-IDE safety and efficacy clinical trial. On May 20, 2000, our patient underwent a successful posterior leaflet quadrangular resection with implantation an annuloplasty band (Figure 14A-14C) (54). She is alive and healthy twenty-two years later and now is ninety-two-year-old. After nineteen additional operative successes, we began a ten-center Multi-institutional Trial, which, eventuated in FDA approval on November 12, 2002, for intra-cardiac use (55). That study showed no to trace mitral regurgitation in 92% of patients by echocardiography one month following surgery. There were no deaths or device-related complications. Subsequently, improved models of this system were developed and commercialized as the daVinci S (2005), daVinci Si (2009) and the daVinci XI (2014), which is the latest device in use today (Figure 15, Figure 16A,16B).
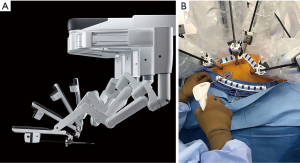
The daVinci™ surgical system today
The daVinci XI Surgical System has many improved features over prior models, including a laser targeting system that facilitates instrument cart docking at the operating table. Moreover, instrument arm positioning at the surgical table now can be programed for specific operations in different surgical specialties. A refined clutching mechanism enables frequent hand-position readjustments to maintain an optimal ergonomic attitude with respect to the visual field. The XI robotic EndoWrist™ instrument tips still provide seven degrees of ergonomic freedom with tremor free dexterity for both dominant and non-dominant hands. These wrist-like micro-instrument articulations improve dexterity in tight spaces. The newer instruments are smaller in diameter and now can be automatically engaged into the robot arm sterile adaptors. There are additional “joints” that allow more adaptable instrument arm positioning, effecting less chance of internal and external conflicts. The 3-dimensional high-definition endoscope is markedly improved and smaller in diameter. For the first time, this model provides a robotic stapling device, which has become a major advantage for non-cardiac thoracic surgeons, as they now have control of positioning and activation. As with the previous Si™ model, the XI™ system has dual-console capability that can both facilitate training and enable surgeons to collaborate during complex cases.
Reluctant surgeons and administrators
The evolution of less invasive cardiac surgery is just over twenty-five years (1995 to 2022). In the beginning there was much resistance, as coronary and mitral valve repair operations were established as having excellent long-term outcomes. There were a number of notable surgeon critics, who were either cautious or vehemently opposed to less invasive operations (56,57). They opined that there were many good reasons for not making these operations “more complex”. First of, was the fact that patients recovered from a sternotomy in a relatively short time, despite discomfort and immobility. They knew that these operations would be more difficult for surgeons, and “They just did not believe in the necessity of this change”. Obviously, concerns over patient safety and inferior results would be disquieting factors. Most surgeons just would not take the risk for their patients or themselves to make the transition to less invasive and especially robot-assisted operations. Even after minimally invasive coronary and valve surgery were shown to have merit, the leap toward robot-assisted surgery was an anathema to many.
A major impediment to early adoption was the purchasing price of the daVinci System as well as the per case and maintenance costs. Administrators considered that purchasing one of these devices for a single service—cardiac surgery—was not economically wise. One must remember that there was then and is now—no additive reimbursement for operations alternatively using the robot. Fortunately, the adoption of robotic surgery in urology, gynecology, and non-cardiac thoracic surgery provided the inflexion point that spawned both device evolution and widespread clinical growth.
Robot-assisted coronary surgery: adoption and expansion
See Table 1 for the chronology of early robot-assisted coronary surgery. As mentioned previously, Mohr and Falk performed the first daVinci-assisted coronary artery bypass operation, which was followed shortly thereafter by Loulmet and Carpentier (49,50). Reichenspurner, Damiano, and Mack were the first to use the Zeus surgical robot for this purpose (40). A subsequent prospective multicenter coronary surgery trial using Zeus showed at three months 93% of the ITA to LAD grafts were patent (2001) (58). Argenziano performed daVinci-assisted TECAB in the United States and later reported results from a retrospective multicenter trial of 76 patients (59). At three months, ITA graft angiographic patency was 92% with 91% of patients free from reintervention. Boyd and associates reported eight-year angiographic follow-up in eighty-two patients who had undergone a robot-assisted CABG using either AES0P 3000, Zeus, or daVinci systems. The ITA to LAD graft patency rate was 93.4% (60). Thereafter, the evolution of robot-assisted coronary operations has been slow, albeit that a few surgeons have become experts and reported good to excellent results (61-64).
Table 1
| May 25, 1998—Mohr/Falk—Leipzig |
| First TECAB using Heartport/cardioplegia with daVinci |
| June 26, 1998—Loulmet and Carpentier—Paris |
| Early TECABs using Heartport/cardioplegia with daVinci |
| September 15, 1998—Reichenspurner/Damiano/Mack—Munich |
| First TECAB using Heartport/cardioplegia with Zeus |
| September 12, 1999—USA—Wolf/Michler—Ohio State |
| First ITA harvest in the USA with daVinci |
| September 18, 1999—Boyd—London, Ontario |
| First beating heart TECAB with Zeus |
| December 9, 1999—United States—Damiano—Hershey |
| First beating heart TECAB in USA with Zeus |
| March 27, 2000—Cichon, Kappert and Gulielmos—Dresden |
| First beating heart TECAB with daVinci |
TECAB, totally endoscopic coronary bypass; ITA, internal thoracic artery.
Recently, Bonatti et al. reviewed seventy-four publications, published between 1996 and 2019, that encompassed 11,135 minimally invasive and/or robotic coronary bypass operations (65). Of these operations, 10.7% were a video-assisted minimally invasive direct coronary bypass (MIDCAB) with 15.8% having an either a robot-assisted MIDCAB or a TECAB (15.5%). The operative mortality for these patients was 1.3% with a stroke rate of 1.0%. The mean operative time for a robotic TECAB was 5.3±0.8 hours. Only 27% were multi-vessel revascularizations and the series had an average of 1.2 grafts per operation. Additionally, there was a 10.3% conversion rate to a sternotomy. In reality, the hospital length of stay for this group was no less that most comparative sternotomy operations.
The predictive future of this surgical modality, no doubt will require new anastomotic device innovations as well as the ingenuity of young cardiac surgeons and engineers.
Balkhy, one of the major proponents of robot-assisted TECAB, has perhaps given us a glimpse of what is possible. His recent publications provide evidence that this operation is feasible being safe, efficacious, and with long-term graft patency. Recently, he reported 570 patients who underwent a robotic TECAB operation (66). His results were excellent with an overall operative mortality of 0.6%. He compared two robotic anastomotic techniques—one cohort had a mechanically stapled anastomosis, using the Cardica C-Port™ Flex-A™ device and in the other group they were done by a “sewn” technique. To render the target site relatively immobile, the Intuitive Coronary Artery Stabilizer was used in all operations. There was no difference in operative complications or anastomotic patency between groups (98% vs. 95%, respectively), but anastomotic times were much longer using the “sewn” technique. This was a glimpse of what is possible, but this guiding light faded when both the robotic coronary stabilizer and anastomotic stapler were withdrawn from commercial sales. As Dr. Balkhy said in his publication, “Until now no other technology has provided enough support for a TECAB operation.” He also stated emphatically, “Finally, for this to happen there needs to be a rapprochement with industry and a mutual commitment to research and development in the field of robotic cardiac surgical instrumentation, especially as it relates to TECAB” and summarized by writing, “Robotic TECAB is in a now or never moment”.
Robot-assisted mitral valve surgery: adoption and expansion
Table 2 lists the early chronological advances in robot-assisted mitral valve repair.
Table 2
| May 7, 1998—Carpentier/Loulmet—Paris |
| First totally computer-assisted mitral repair using daVinci |
| May 23, 1998—Mohr/Falk—Leipzig |
| Totally computer-assisted mitral repairs (n=5) using daVinci |
| March 2000—Lange—Munich |
| First totally endoscopic (ports only) robotic mitral repair using daVinci |
| May 2000—Grossi—New York |
| First mitral leaflet repair in North America—Zeus |
| May 20, 2000—Chitwood/Nifong/Elbeery—ECU |
| First complete mitral repair (with annuloplasty) in North America—daVinci |
At first adoption of robotic mitral valve surgery in the United States was very slow, even after the first successful FDA clinical trials, and was almost non-existent in Europe. As mentioned, in 1998 Carpentier (Paris) and Mohr (Leipzig) performed the first robot-assisted mitral valve repairs. Later, both the New York University surgeons and our group performed the first of these operations in the United States. Had these early operations failed, cardiac surgical robotics may never have developed.
To illustrate the skepticism of those times, the following excerpts are from an editorial-based dialog between the author and Dr. Francis Robicsek. In 2003 he wrote, “To justify the introduction of this complex and certainly most expensive technology … So far, I have found none. … What can cardiac robotics offer that other simpler and less expensive techniques cannot?” (67). Then, I rejoined, “Well, Dr. Robicsek, we meet again! It is interesting to me that you—one who has been such an innovator and so progressive in cardiac surgery—now are so skeptical of robotic cardiac surgery. Sir, give robotic surgery a chance to develop and time will prove either the value or folly.” (68). Four years later in his Time told! editorial, he wrote: “Today, cardiac surgeons, with the exception of a few “forever” enthusiasts such as Chitwood, seem to be less interested in robotic technology than they were 5 years ago.” (69).
Well, time did tell! Although no randomized trials have been done, comparing robot-assisted and non-robotic minimally invasive to traditional mitral operations, many large clinical series have shown excellent outcomes with the latter operative method. In our inaugural series of 540 patients, 84% underwent a lone mitral repair and 16% had a concomitant bi-atrial Cox cryothermic ablation (70). The majority of operations were leaflet resections followed by an annuloplasty, and 98% had either no or trace mitral insufficiency following the operation. The overall 30-day mortality was 0.2% for those having only a mitral repair, and there were no device-related complications. Since that time, a number of large single center series have been published and are shown in Table 3 (71-76). In these six publications, 4,745 robot-assisted mitral operations were reported with a mortality of between 0.1% and 0.9% and a stroke rate of 0.7% to 2.2%. Because of the combination of better preoperative screening, patient selection, surgical robot advancement, and team experience, most programs have experienced a constant improvement in operative efficiency and clinical outcomes. In our inaugural robotic mitral repair series, we thought our selection process was strict because of the initial FDA trials. Although our results were good, a more rigorous selection process now has been shown to be of significant benefit. Recently, the Cleveland Clinic group compared results in their first 500 robotic mitral surgery patients, who were not screened radiographically, to the next 500 cohort, who were rigorously CT studied preoperatively (77). They showed a marked reduction in neurological events in the later patients. Thereafter, their selection algorithm included vascular CT screening on all patient candidate and excluded those with other contraindications.
Table 3
| Series | Number of repairs | DMR | FMR | Mortality | Stroke | Maze | Reoperation | MR, mild or less |
|---|---|---|---|---|---|---|---|---|
| Dearani (71) | 834 | 100% | 0% | 0.4% | 0.8% | 6% | 2.8% | 98% |
| Loulmet (72) | 500 | 76% | 7% | 0.6% | 1.2% | 19% | – | 99% |
| Chitwood (73) | 944 | 94% | 4% | 0.2% | 1.3% | 47% | 2.5% | 97% |
| Murphy (74) | 1,167 | 88% | 5% | 0.9% | 0.7% | 18% | 3.9% | 98% |
| Gillinov (75) | 1000 | 96% | 1% | 0.1% | 2.2% | 7% | 2.3% | 98% |
| Trento (76) | 300 | 100% | 0% | 0.3% | 1.7% | 22% | 3.0% | 84% |
*, from top to bottom series are listed by most recent publication with reference numbers. DMR, degenerative mitral regurgitation; FMR, functional mitral regurgitation; Mortality, 30-day post operative; Reoperation, at various intervals; MR, mitral regurgitation, leaving the operating room or hospital.
After reviewing the publications shown in Table 3, the author was impressed by the varied mitral valve pathology selected for these operations. As expected, there was a preponderance (94% to 100%) of patients having degenerative mitral regurgitation. Nevertheless, major reference centers are doing very complex operations including patients with advanced Barlow’s pathology, mitral annular calcification, and healed endocarditis. Loulmet and Grossi robotically performed simple, complex, and most complex repairs in 48%, 28%, 24% of their patients, respectively (72). Among these groups, the number of required repair techniques escalated from 2.8 to 3.9 to 5.3, respectively. Their publication describes the myriad of repair techniques that should be considered for the robotic mitral surgeon’s “toolbox”. Results have been excellent even in patients presenting with a prior sternotomy (78,79).
It is not within the scope of this report to compare “head-to-head” other approaches for mitral valve surgery to individual robot-assisted series. However, the evidence today clearly shows equivalency in not only safety but also in repair quality. Recently, surgeons of the Virginia Cardiac Services Quality Initiative published an analysis of 2,351 patients from nineteen centers, who had either a robotic (n=372), minimally invasive (n=576) or conventional (n=1,352) mitral operation (80). Of these, 628 were propensity matched. Repair rates were higher in the robotic group (91%) versus conventional operations (76%). Overall, results from robot-assisted operations were similar to the other two modalities with exception of a lower mortality in the former at 0.8% with an O/E ratio of 0.62. This study confirms safety and efficacy of robotic mitral operations, however, comparisons to the other two modalities may have been confounded by patient selection. Another study by Paul et al. reviewed 3,145 patients that had a robot-assisted mitral valve repair (81). They subsequently compared 631 propensity matched patients to those with a non-robotic operation and found hospital mortality, complications, and clinical outcomes were similar. Moreover, hospitalization was two days less and overall economic costs were the same.
Early criticisms of robot-assisted cardiac surgery were that the cardiopulmonary perfusion and cross clamp times would be too long to be safe. Indeed, in most series these are longer that operating through a full sternotomy. However, the evidence has shown most of the time these have not impacted overall safety, especially in patients with less complex mitral anatomy and good ventricular function. Additionally, the economic concerns have been quelled by several groups, who investigated all aspects of the clinical expense chain (82-84). They have shown that by closely evaluating and “process re-engineering” each incremental part of the patient care pathway, that costs can be reduced. Most of these patients are reasonably healthy other than the mitral malady. Thus, extubation in the operating room de facto decreases residence in intensive care unit and may result in early hospital discharge.
Robot-assisted aortic valve surgery: re-emerging
On March 7, 2005, Folliguet performed the first robot-assisted aortic valve replacement through a right anterior mini-thoracotomy (85). Later, he reported five additional cases with no deaths or major complications. In 2020, Balkhy reported implantation of the sutureless Percival aortic valve robotically (86). Recently, Wei and Badhwar “reignited” robotic aortic valve replacement by reporting 50 cases with no deaths or other major complications (87). Their technique was unique in that they used the same lateral mini thoracotomy as in their robotic mitral operations. With time this method may become a new standard for a surgical aortic valve replacement.
The team! The training!
To have a successful cardiac surgical robotic program adequate team training, and good clinical volume are essential. Our Multi-specialty Robotic Surgical Training Laboratory at East Carolina University (1999) was the first in this country to train surgeons formally in clinical robotics. The “hub” of the curriculum consisted of daVinci system training, which was followed by specific procedure training, case observation, and proctoring of early operations at the surgeon mentee’s institution (88). To track technical progress, keeping metric data was emphasized. Later, procedure simulation was added after the daVinci “backpack” simulator was developed. Over the years we refined and modified our educational format (89). Badhwar et al. recently detailed the latest information regarding contemporary robotic cardiac surgical training and emphasized requisites to establish a successful institutional program (90). The publication by Rodriguez et al. describes the best pathway to establish and maintain a successful cardiac robotic program (91). All of these authors continually have focused on robotic team training and development as operative synchrony is imperative to have optimal outcomes.
The Society for Thoracic Surgeons (STS) with Intuitive Surgical Inc. sponsors a two-day training course in robot-assisted coronary and mitral valve surgery. In addition, recently both the STS and American Association for Thoracic Surgery offer training fellowships for surgeons experienced in mitral valve repair. Similarly, the European Association for Thoracic and Cardiovascular Surgery has just launched the Fontan Fellowship for training cardiothoracic surgeons in this subspecialty. Thus, today we are seeing an ever-expanding interest in robotic surgery for both cardiac and non-cardiac thoracic surgery both in the United States and Europe (92).
Concluding comments
“It is not the strongest of the species that survives, nor the most intelligent that survives. It is the one that is most adaptable to change …”
—Charles Darwin
Unfortunately, this edition of the Annals of Cardiothoracic Surgery could not “bare the weight” of including all the relevant contributors and their accomplishments. Additionally, there have been many omissions, including robotic ablation for atrial fibrillation, atrial septal defect closure, cardiac tumor extirpation, septal myomectomy, or hybrid coronary procedures. As Julius Caesar once said about crossing the Rubicon River, “iacta alea est” (The die is cast). There is no turning back! For sure, the technologic trajectory for robotic surgery is advancing rapidly, and the number of surgeon adopters is ever increasing. Also, several new surgical robots are in development and will be discussed by other contributors to this edition of Annals. Will the future device generations have artificial intelligence driven automation, robot diagnostic imaging coupling, nanomotor driven miniaturized instruments, or operative data registration in simulators? To the next generation of surgeons and their patients, we now deliver to you the vision and successes of both the pioneers and modern contributors. It is incumbent that young surgeon innovators carry this journey to a new destination, which will provide the very least invasive surgical care for their patients. Charles Darwin’s quote alone bespeaks the message that adaption to change is imperative to advance our specialty.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author declares no conflicts of interest.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Čapek K. Rossumovi Univerzální Roboti (Rossum’s Universal Robots). Prague, 1921.
- Kolessov VI. Mammary artery-coronary artery anastomosis as method of treatment for angina pectoris. J Thorac Cardiovasc Surg 1967;54:535-44. [Crossref] [PubMed]
- Ankeny JL. Editorial: To use or not to use the pump oxygenator in coronary bypass operations. Ann Thorac Surg 1975;19:108-9. [Crossref] [PubMed]
- Ankeney JL, Goldstein DJ. Off-pump bypass of the left anterior descending coronary artery: 23- to 34-year follow-up. J Thorac Cardiovasc Surg 2007;133:1499-503. [Crossref] [PubMed]
- Buffolo E, de Andrade CS, Branco JN, et al. Coronary artery bypass grafting without cardiopulmonary bypass. Ann Thorac Surg 1996;61:63-6. [Crossref] [PubMed]
- Ullyot DJ. Look ma, no hands! Ann Thorac Surg 1996;61:10-1. [Crossref] [PubMed]
- Benetti FJ, Ballester C, Barnia YA. Uso de la Toracoscopía en cirugía coronaria para disección de la mamaria izquierda. La Prensa Médica Argentina 1994;9:81-7.
- Benetti FJ, Ballester C. Use of thoracoscopy and a minimal thoracotomy, in mammary-coronary bypass to left anterior descending artery, without extracorporeal circulation. Experience in 2 cases. J Cardiovasc Surg (Torino) 1995;36:159-61. [PubMed]
- Calafiore AM, Giammarco GD, Teodori G, et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann Thorac Surg 1996;61:1658-63; discussion 1664-5. [Crossref] [PubMed]
- Nataf P, Lima L, Benarim S, et al. Video-assisted coronary bypass surgery: clinical results. Eur J Cardiothorac Surg 1997;11:865-9. [Crossref] [PubMed]
- Mack M, Acuff T, Yong P, et al. Minimally invasive thoracoscopically assisted coronary artery bypass surgery. Eur J Cardiothorac Surg 1997;12:20-4. [Crossref] [PubMed]
- Borst C, Jansen EW, Tulleken CA, et al. Coronary artery bypass grafting without cardiopulmonary bypass and without interruption of native coronary flow using a novel anastomosis site restraining device ("Octopus"). J Am Coll Cardiol 1996;27:1356-64. [Crossref] [PubMed]
- Puskas J, Cheng D, Knight J, et al. Off-Pump versus Conventional Coronary Artery Bypass Grafting: A Meta-Analysis and Consensus Statement From The 2004 ISMICS Consensus Conference. Innovations (Phila) 2005;1:3-27. [Crossref] [PubMed]
- Pompili MF, Stevens JH, Burdon TA, et al. Port-access mitral valve replacement in dogs. J Thorac Cardiovasc Surg 1996;112:1268-74. [Crossref] [PubMed]
- Stevens JH, Burdon TA, Peters WS, et al. Port-access coronary artery bypass grafting: a proposed surgical method. J Thorac Cardiovasc Surg. 1996;111:567-573. [Crossref] [PubMed]
- Pompili MF, Yakub A, Siegel LC, et al. Port-access mitral valve replacement: initial clinical experience. Circulation 1996;94:I-533.
- Navia JL, Cosgrove DM 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg 1996;62:1542-4. [Crossref] [PubMed]
- Aklog L, Adams DH, Couper GS, et al. Techniques and results of direct-access minimally invasive mitral valve surgery: a paradigm for the future. J Thorac Cardiovasc Surg 1998;116:705-15. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23. [PubMed]
- Benetti FJ, Rizzardi JL, Pire L, et al. Mitral valve replacement under video assistance through a minithoracotomy. Ann Thorac Surg 1997;63:1150-2. [Crossref] [PubMed]
- Personal communication with Michael Pankratov, PhD. September 2022.
- Westaby S, Benetti FJ. Less invasive coronary surgery: consensus from the Oxford meeting. Ann Thorac Surg 1996;62:924-31. [Crossref] [PubMed]
- Chitwood WR Jr, Elbeery JR, Moran JF. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg 1997;63:1477-9. [Crossref] [PubMed]
- Chitwood WR Jr, Wixon CL, Elbeery JR, et al. Video-assisted minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg 1997;114:773-80; discussion 780-2. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [Crossref] [PubMed]
- Baldwin JC. Editorial (con) re minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:563-4. [Crossref] [PubMed]
- Vanermen H, Farhat F, Wellens F, et al. Minimally invasive video-assisted mitral valve surgery: from Port-Access towards a totally endoscopic procedure. J Card Surg 2000;15:51-60. [Crossref] [PubMed]
- Reichenspurner H, Detter C, Deuse T, et al. Video and robotic-assisted minimally invasive mitral valve surgery: a comparison of the Port-Access and transthoracic clamp techniques. Ann Thorac Surg 2005;79:485-90; discussion 490-1. [Crossref] [PubMed]
- Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [Crossref] [PubMed]
- Modi P, Rodriguez E, Hargrove WC 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7. [Crossref] [PubMed]
- Ward AF, Grossi EA, Galloway AC. Minimally invasive mitral surgery through right mini-thoracotomy under direct vision. J Thorac Dis. 2013;5:S673-9. [PubMed]
- Barac YD, Glower DD. Port-Access Mitral Valve Surgery-An Evolution of Technique. Semin Thorac Cardiovasc Surg 2020;32:829-37. [Crossref] [PubMed]
- Balkhy HH, Grossi EA, Liaii B, et al. Retrospective evaluation of endo-aortic balloon occlusion compared to external clamping in minimally invasive mitral valve surgery. Seminars in Thoracic and Cardiovascular Surgery. (in press;
- Rosen J, Hannaford B, Satava RM. Editors. Surgical Robotics: Systems Applications and Visions. New York: Springer; 2010.
- George EI, Brand TC, LaPorta A, et al. Origins of Robotic Surgery: From Skepticism to Standard of Care. JSLS 2018;22:e2018. [Crossref] [PubMed]
- Green PS. Telepresence: dexterous procedures in a virtual operating field. Surg Endosc 1991;57:192.
- Pugin F, Bicher P, Morel P. History of robotic surgery: From AESOP and ZEUS to daVinci. J Visc Surg 2011;148:e3-8. [Crossref] [PubMed]
- Bowersox JC, Shah A, Jensen J, et al. Vascular applications of telepresence surgery: initial feasibility studies in swine. J Vasc Surg 1996;23:281-7. [Crossref] [PubMed]
- Falk V, Walther T, Autschbach R, et al. Robot-assisted minimally invasive solo mitral valve operation. J Thorac Cardiovasc Surg 1998;115:470-1. [Crossref] [PubMed]
- Reichenspurner H, Damiano RJ, Mack M, et al. Use of the voice-controlled and computer-assisted surgical system ZEUS for endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg 1999;118:11-6. [Crossref] [PubMed]
- Boyd WD, Rayman R, Desai ND, et al. Closed-chest coronary artery bypass grafting on the beating heart with the use of a computer-enhanced surgical robotic system. J Thorac Cardiovasc Surg 2000;120:807-9. [Crossref] [PubMed]
- Grossi EA, Chen S, Loulmet DF. Commentary: Robotic Techniques in Cardiac and Thoracic Surgery (Innovations, May/June 2020). Innovations (Phila) 2020;15:423-4. [Crossref] [PubMed]
- Marescaux J, Leroy J, Gagner M, et al. Transatlantic robot-assisted telesurgery. Nature. 2001;413:379-80. Erratum in: Nature 2001;414:710. [Crossref] [PubMed]
- Marescaux J, Leroy J, Rubino F, et al. Transcontinental robot-assisted remote telesurgery: feasibility and potential applications. Ann Surg 2002;235:487-92. [Crossref] [PubMed]
- Shennib H, Bastawisy A, Mack MJ, et al. Computer-assisted telemanipulation: an enabling technology for endoscopic coronary artery bypass. Ann Thorac Surg 1998;66:1060-3. [Crossref] [PubMed]
- Himpens J, Leman G, Cadiere GB. Telesurgical laparoscopic cholecystectomy. Surg Endosc 1998;12:1091. [Crossref] [PubMed]
- Carpentier A, Loulmet D, Aupècle B, et al. Computer assisted open heart surgery. First case operated on with success. C R Acad Sci III 1998;321:437-42. [Crossref] [PubMed]
- Autschbach R, Onnasch JF, Falk V, et al. The Leipzig experience with robotic valve surgery. J Card Surg 2000;15:82-7. [Crossref] [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Computer-enhanced coronary artery bypass surgery. J Thorac Cardiovasc Surg 1999;117:1212-4. [Crossref] [PubMed]
- Loulmet D, Carpentier A, d'Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [Crossref] [PubMed]
- Falk V, Diegeler A, Walther T, et al. Total endoscopic computer enhanced coronary artery bypass grafting. Eur J Cardiothorac Surg 2000;17:38-45. [Crossref] [PubMed]
- Kappert U, Cichon R, Schneider J, et al. Closed-chest coronary artery surgery on the beating heart with the use of a robotic system. J Thorac Cardiovasc Surg 2000;120:809-11. [Crossref] [PubMed]
- Personal Communication with Robert Michler, MD. September 2022.
- Chitwood WR Jr, Nifong LW, Elbeery JE, et al. Robotic mitral valve repair: trapezoidal resection and prosthetic annuloplasty with the da vinci surgical system. J Thorac Cardiovasc Surg 2000;120:1171-2. [Crossref] [PubMed]
- Nifong LW, Chitwood WR, Pappas PS, et al. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg 2005;129:1395-404. [Crossref] [PubMed]
- Cooley DA. Antagonist's view of minimally invasive heart valve surgery. J Card Surg 2000;15:3-5. [Crossref] [PubMed]
- Lytle BW. Minimally invasive cardiac surgery. J Thorac Cardiovasc Surg 1996;111:554-5. [PubMed]
- Damiano RJ Jr, Tabaie HA, Mack MJ, et al. Initial prospective multicenter clinical trial of robotically-assisted coronary artery bypass grafting. Ann Thorac Surg 2001;72:1263-8; discussion 1268-9. [Crossref] [PubMed]
- Argenziano M, Katz M, Bonatti J, et al. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg 2006;81:1666-74; discussion 1674-5. [Crossref] [PubMed]
- Currie ME, Romsa J, Fox SA, et al. Long-term angiographic follow-up of robotic-assisted coronary artery revascularization. Ann Thorac Surg 2012;93:1426-31. [Crossref] [PubMed]
- de Cannière D, Wimmer-Greinecker G, Cichon R, et al. Feasibility, safety, and efficacy of totally endoscopic coronary artery bypass grafting: multicenter European experience. J Thorac Cardiovasc Surg 2007;134:710-6. [Crossref] [PubMed]
- Srivastava S, Gadasalli S, Agusala M, et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg 2010;89:1873-9; discussion 1879-80. [Crossref] [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Robotically assisted totally endoscopic coronary bypass surgery. Circulation 2011;124:236-44. [Crossref] [PubMed]
- Bonaros N, Schachner T, Lehr E, et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg 2013;95:803-12. [Crossref] [PubMed]
- Bonatti J, Wallner S, Crailsheim I, et al. Minimally invasive and robotic coronary artery bypass grafting-a 25-year review. J Thorac Dis 2021;13:1922-44. [Crossref] [PubMed]
- Balkhy HH, Nisivaco SM, Hashimoto M, et al. Robotic Total Endoscopic Coronary Bypass in 570 Patients: Impact of Anastomotic Technique in Two Eras. Ann Thorac Surg 2022;114:476-82. [Crossref] [PubMed]
- Robicsek F. Robotic cardiac surgery: quo vadis? J Thorac Cardiovasc Surg 2003;126:623-4. [Crossref] [PubMed]
- Chitwood WR Jr. An epistle to Dr Robicsek. J Thorac Cardiovasc Surg 2004;127:945-6. [Crossref] [PubMed]
- Robicsek F. Robotic cardiac surgery: time told! J Thorac Cardiovasc Surg 2008;135:243-6. [Crossref] [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [Crossref] [PubMed]
- Arghami A, Jahanian S, Daly RC, et al. Robotic Mitral Valve Repair: A Decade of Experience With Echocardiographic Follow-up. Ann Thorac Surg 2022;114:1587-95. [Crossref] [PubMed]
- Loulmet DF, Ranganath NK, Neuburger PJ, et al. Can complex mitral valve repair be performed with robotics? An institution's experience utilizing a dedicated team approach in 500 patients. Eur J Cardiothorac Surg 2019;56:470-8. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 2016;5:544-55. [Crossref] [PubMed]
- Murphy DA, Moss E, Binongo J, et al. The Expanding Role of Endoscopic Robotics in Mitral Valve Surgery: 1,257 Consecutive Procedures. Ann Thorac Surg 2015;100:1675-81; discussion 1681-2. [Crossref] [PubMed]
- Gillinov AM, Mihaljevic T, Javadikasgari H, et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J Thorac Cardiovasc Surg 2018;155:82-91.e2. [Crossref] [PubMed]
- Ramzy D, Trento A, Cheng W, et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228-35. [Crossref] [PubMed]
- Chemtob RA, Wierup P, Mick SL, et al. A conservative screening algorithm to determine candidacy for robotic mitral valve surgery. J Thorac Cardiovasc Surg 2022;164:1080-7. [Crossref] [PubMed]
- Meidan TG, Lanfear AT, Squiers JJ, et al. Robotic mitral valve surgery after prior sternotomy. JTCVS Tech 2022;13:46-51. [Crossref] [PubMed]
- Murphy DA, Jonsson AA, Halkos ME. Endoscopic Robotic Mitral Valve Surgery in Patients With Previous Sternotomy Cardiac Surgery. Innovations (Phila) 2022;17:297-303. [Crossref] [PubMed]
- Hawkins RB, Mehaffey JH, Mullen MG, et al. A propensity matched analysis of robotic, minimally invasive, and conventional mitral valve surgery. Heart 2018;104:1970-5. [Crossref] [PubMed]
- Paul S, Isaacs AJ, Jalbert J, et al. A population-based analysis of robotic-assisted mitral valve repair. Ann Thorac Surg 2015;99:1546-53. [Crossref] [PubMed]
- Suri RM, Thompson JE, Burkhart HM, et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin Proc 2013;88:1075-84. [Crossref] [PubMed]
- Mihaljevic T, Koprivanac M, Kelava M, et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg 2014;149:679-86. [Crossref] [PubMed]
- Coyan G, Wei LM, Althouse A, et al. Robotic mitral valve operations by experienced surgeons are cost-neutral and durable at 1 year. J Thorac Cardiovasc Surg 2018;156:1040-7. [Crossref] [PubMed]
- Folliguet TA, Vanhuyse F, Konstantinos Z, et al. Early experience with robotic aortic valve replacement. Eur J Cardiothorac Surg 2005;28:172-3. [Crossref] [PubMed]
- Balkhy HH, Kitahara H. First Human Totally Endoscopic Robotic-Assisted Sutureless Aortic Valve Replacement. Ann Thorac Surg 2020;109:e9-11. [Crossref] [PubMed]
- Wei LM, Cook CC, Hayanga JWA, et al. Robotic Aortic Valve Replacement: First 50 Cases. Ann Thorac Surg 2022;114:720-6. [Crossref] [PubMed]
- Chitwood WR Jr, Nifong LW, Chapman WH, et al. Robotic surgical training in an academic institution. Ann Surg 2001;234:475-84; discussion 484-6. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic Mitral Valve Repair: How I Teach It. Ann Thorac Surg 2019;107:1297-301. [Crossref] [PubMed]
- Badhwar V, Wei LM, Geirsson A, et al. Contemporary robotic cardiac surgical training. J Thorac Cardiovasc Surg 2021;S0022-5223(21)01541-5. Epub ahead of print. [Crossref] [PubMed]
- Rodriguez E, Nifong LW, Bonatti J, et al. Pathway for Surgeons and Programs to Establish and Maintain a Successful Robot-Assisted Adult Cardiac Surgery Program. Ann Thorac Surg 2016;102:340-4. [Crossref] [PubMed]
- Cerny S, Oosterlinck W, Onan B, et al. Robotic Cardiac Surgery in Europe: Status 2020. Front Cardiovasc Med 2021;8:827515. [Crossref] [PubMed]







