Safety of cerebrospinal fluid drainage in descending and thoracoabdominal aortic replacement surgery
Introduction
Spinal cord injury (SCI) is a multifactorial complication of surgical repair of descending thoracic aortic aneurysms (DTAA) and thoracoabdominal aortic aneurysms (TAAA) (1), with resulting paraplegia and paraparesis severely impacting the quality of a patient’s life, as well as markedly diminishing early and late survival (2). Spinal cord ischemia and infarction result from disruption of the blood supply (3) to the anterior spinal cord, with aortic cross-clamping a significant contributor to this pathology (Figure 1). Although it was anticipated that incidence of paraplegia would diminish with the advent of endovascular therapies, this has not been borne out. In fact, a specific profile of paraplegia unique to endovascular therapies has emerged (5). Additionally, with the increased total number of cases being done in the current endovascular era, the total number of cases of peri-operative paraplegia is thought to be increasing (6).
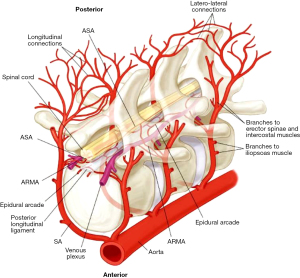
Prevention of SCI requires appropriate pre-operative risk assessment for the patients, as well as employing a combination of protective strategies peri- and postoperatively to ensure adequate perfusion of the spinal cord. These treatment adjuncts may include cerebrospinal fluid drainage (CSFD), motor evoked potential monitoring, left heart bypass, epidural cooling, and preoperative imaging to identify and preserve the artery of Adamkiewicz (7-11). The American College of Cardiology and American Heart Association designates a class IA level recommendation (12) for the use of CSFD for decreasing the incidence of temporary, as well as permanent SCI, in patients undergoing open thoracoabdominal aortic repair. However, the use of a cerebrospinal fluid (CSF) drain is not without complications of its own, which can range from post-dural puncture headache (13) and puncture-site bleeding through to subdural hematoma, amongst others (14,15). Herein, we present our 17-year experience using a spinal drain as a protective strategy against SCI during open descending and thoracoabdominal aortic repairs.
Methods
The database of the Aortic Institute at Yale New Haven was queried to retrieve information for this study. Supplementary EPIC chart review was conducted as needed. This study was approved by the Human Investigation Committee of the Yale University School of Medicine. Patients’ informed consent was waived due to the study being a medical record review. This study expands on a prior report from our institution (8). Patients undergoing endovascular repair of the aorta, as well as patients receiving open repair without a CSF drain, were excluded from the study. At our institute, we consistently use a CSF drain for open repair of DTAA/TAAA unless contraindicated or unable to be placed.
The intervention of interest for this study was the placement and appropriate use of CSF drain. Spinal drain insertion was primarily performed at three specific lumbar levels: L4–L5, L3–L4, and L2–L3. There were two different approaches utilized for placing the CSFD catheter: one involved the patient being awake and in a sitting position, while the other approach took place after anesthesia induction with the patient lying on their side. The procedure employed a CSFD kit from Medtronic Neurosurgery (model 46419) that included a Tuohy needle (14-gauge, 9 cm with Huber tip), a 20-gauge blunt needle, and a guide wire. Additionally, a Duet External Drainage and Monitoring System with Baxter Interlink needleless injection sites, along with an 80-cm closed-tipped EDM lumbar drainage catheter (all from Medtronic, Minneapolis, MN, USA) were used. During the procedure, the Tuohy needle was inserted at the designated level following standard landmarks until clear CSF fluid was obtained. The catheter was then introduced approximately 6 cm beyond the needle’s tip. In cases where the patient experienced paresthesia during needle insertion, the advancement of the needle was halted and the needle withdrawn around 5 mm until the patient confirmed complete resolution of the abnormal sensations. Afterwards, the needle was redirected and advanced, ensuring the absence of paresthesia or pain. In one specific patient who had a complex history of four previous lumbar spine operations, challenges were encountered during the placement of the spinal drain. After multiple attempts, CSF was successfully obtained using an L2 paramedian approach. On average, the CSFD was left in place for a duration of 2.3±0.7 days. The objective was to maintain CSF pressure below 10 mmHg throughout the surgical procedure by draining CSF at a rate of 20 to 30 mL/h whenever necessary. Following the surgery, pressure was maintained within the range of 5 to 12 mmHg.
In our practice, it is our standard policy to utilize a spinal drain for all cases involving open descending and thoracoabdominal aortic procedures, irrespective of their extent. However, there are exceptional circumstances where the placement of a CSFD may not be possible due to anatomical or patient comorbidity related reasons, such as prior spinal fusion. In such cases, we proceed with the surgery without the drain. In situations where there is a clear rupture of the aorta accompanied by hemodynamic instability, we proceed with the surgery without implementing a drain. As part of our protocol, we consistently remove the lumbar drain on the morning of the second postoperative day. The only situation in which we deviate from this practice is when there is a presence of neurologic impairment or any related concerns.
In addition to CSFD, we also employ several concurrent peri- and intraoperative protective strategies aimed at reducing the occurrence of SCI (16). Our standard approach includes utilization of dual-energy CT scan to identify the origin of the spinal artery pre-operatively. During surgery, we prioritize the preservation of intercostal arteries whenever feasible, use a spinal drain as a routine practice, as well as left atrial-femoral artery bypass with an oxygenator. We also employ and monitor motor evoked potentials intraoperatively. Postoperatively, we maintain systolic blood pressure above 125 mmHg, starting from 2 hours onwards. The impact of these various protective measures utilized throughout our clinical experience cannot be individually discerned, as they were implemented collectively. As part of our routine practice, we frequently use a pulmonary artery (Swan-Ganz) catheter. In terms of fluid management, we aim to return adequate intravenous fluid to maintain a balance between urine output and chest tube drainage, as a minimum requirement. Attention is paid to maintaining a hematocrit concentration at or above 30% to ensure adequate oxygen supply to the spinal cord, promote appropriate coagulation, and achieve surgical hemostasis. We do not incorporate any pharmacologic adjuncts, such as glucocorticoids, during the perioperative period.
Our retrospective inquiry yielded 132 patients who underwent open surgical repair of DTAA and TAAA with the insertion of CSF drain for spinal cord protection. During this period, only 4 patients had open surgical management of their aortas without CSF drain placement. We conducted an extensive electronic health record (EHR) review to abstract pertinent information regarding patient demographics, surgical history, and post-operative course as well as survival. Specific attention was applied to the use of adjunct spinal cord protection strategies, including peri-operative anterior spinal artery detection by computed tomography (CT) scan, intraoperative use of left atrial femoral bypass, and monitoring of motor evoked potentials, as well as preserving or reimplanting critical intercostal arteries. Data was computed for all complications of CSFD. Paraplegia status was also assessed and recorded. We utilized Microsoft Excel for Mac (Version 16.72; Redmond, WA, USA) for computation of data, and GraphPad Prism (version 9.00 for MacOS, GraphPad Software, Boston, MA, USA) for analyzing continuous and categorical variables. We assessed survival using Kaplan-Meier analytical method that was visualized using Prism software. Continuous variables are presented as mean and standard deviation, and categorical variables are presented as number and percentage in our results.
Results
The average age of the patients in our study was 65.4±13 years [male n=82 (62.1%), average age 64.8 years, female n=50 (37.9%), average age 66.6 years]. Patient demographics and pre-operative comorbidity distribution are provided in Table 1. Among our cohort, 75 patients (56.8%) had nondissected aneurysms, 53 (40.2%) had dissected aneurysms, and 2 (1.5%) presented with contained aneurysm rupture. Only 2 (1.5%) patients had an acute or subacute type B dissection. Overall, only 10 (7.6%) operations were carried out on urgent basis.
Table 1
| Characteristic/variable | Value |
|---|---|
| Age at the time of surgery, year | 65.4±13.0 |
| Male sex | 82 (62.1) |
| Height, cm | 172.2±11.1 |
| Weight, kg | 81.6±20 |
| Body mass index, kg/m2 | 27.3±5.3 |
| Hypertension | 129 (97.7) |
| Diabetes | 22 (16.7) |
| Dyslipidemia | 77 (58.3) |
| Obesity | 43 (32.6) |
| Chronic obstructive pulmonary disease | 37 (28.0) |
| Chronic kidney disease | 18 (13.6) |
| Coronary artery disease | 54 (40.9) |
| Marfan syndrome | 13 (9.8) |
| History of smoking | 92 (69.9) |
| Previous cardiac surgery (nonaortic) | 38 (28.8) |
| Previous aortic surgery in proximal or distal segments | 90 (68.2) |
| Confirmed family history of aortic disease | 32 (24.2) |
Data are presented as n (%) and mean ± standard deviation.
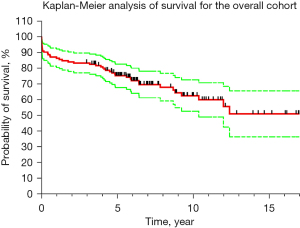
Out of the total, 48 patients (36.4%) had a DTAA, and 84 (63.6%) had a TAAA. According to the Safi modification of the Crawford aneurysm classification, the distribution was as follows: 25 patients (18.9%) were classified as type I, 38 (28.8%) as type II, 12 (9.1%) as type III, 3 (2.3%) as type IV, and 6 (4.5%) as type V. Surgical repair involved the descending aorta in 66 patients (50.0%), the thoracoabdominal aorta in 65 patients (49.2%), and a combined replacement of the aortic arch and descending aorta in 1 patient (0.8%). Furthermore, 43 operations (32.6%) were performed with a distal completion of a previously inserted elephant trunk (Table 2). The specific spinal cord protection strategies applied individually to each patient based on the surgeon’s discretion and tailored to their unique circumstances are enlisted in Table 3. To evaluate the survival rates, we employed the Yale Aortic Institute survival assessment technique (17) and utilized the Kaplan-Meier analytical method (refer to Figure 2). The overall cohort demonstrated the following survival rates: 86.4% at 1 year, 83.3% at 3 years, 75.2% at 5 years, 62.6% at 10 years, and 50.9% at 15 years.
Table 2
| Parameter | N (%) |
|---|---|
| Nondissected aneurysm | 75 (56.8) |
| Dissected aneurysm | |
| Total | 53 (40.2) |
| Type A dissection | 18 (13.6) |
| Type B dissection | 35 (26.5) |
| Contained rupture of aneurysm | 2 (1.5) |
| Intramural hematoma of descending aorta | 1 (0.8) |
| Deep penetrating ulcer of descending aorta | 1 (0.8) |
| Dissection | |
| Acute type A | 1 (0.8) |
| Chronic type A | 17 (12.9) |
| Acute/subacute type B | 5 (3.8) |
| Chronic type B | 30 (22.7) |
| Location of aortic pathology | |
| Descending aorta | 48 (36.4) |
| Thoracoabdominal aorta | 84 (63.6) |
| Crawford extent of TAAA | |
| Type I | 25 (18.9) |
| Type II | 38 (28.8) |
| Type III | 12 (9.1) |
| Type IV | 3 (2.3) |
| Type V | 6 (4.5) |
| Extent of surgical repair | |
| Combined arch and descending aorta | 1 (0.8) |
| Descending aorta | 66 (50.0) |
| Thoracoabdominal aorta | 65 (49.2) |
| Operations performed with distal completion of a previously inserted elephant trunk (stage II elephant trunk) via left thoracotomy | 43 (32.6) |
| Operation | |
| Elective | 122 (92.4) |
| Urgent | 10 (7.6) |
TAAA, thoracoabdominal aortic aneurysm.
Table 3
| Protection strategy | N [%] |
|---|---|
| Preserving intercostals | 92 [70] |
| Motor evoked potentials | 87 [66] |
| Anterior spinal artery detected before surgery | 96 [73] |
| Left atrial-femoral bypass | 125 [95] |
Among the patients in our study, a total of 10 (7.6%) experienced in-hospital postoperative mortality. Of these, two (1.5%) died intraoperatively. Another two (1.5%) passed away within 30 days after discharge from the hospital. Table 4 provides an overview of mortality and major postoperative complications categorized by the type of aneurysm and Crawford extent. The majority of complications occurred among patients with extensive aneurysms, specifically Crawford type I and II, followed by the DTAA group.
Table 4
| Parameter | DTAA (N=48) | Type I TAAA (N=25) | Type II TAAA (N=38) | Type III TAAA (N=12) | Type IV TAAA (N=3) | Type V TAAA (N=6) | All patients (N=132) |
|---|---|---|---|---|---|---|---|
| In-hospital mortality (postoperative), n (%) | 2 (1.5) | 3 (2.3) | 4 (3.0) | – | 1 (0.8) | – | 10 (7.6) |
| 30-day mortality (after hospital discharge), n (%) | 1 (0.8) | 1 (0.8) | – | – | – | – | 2 (1.5) |
| Paraplegia, n (%) | 1 (0.8) | 1 (0.8) | 2 (1.5) | – | – | – | 4 (3.0) |
| Transient extremity weakness, n (%) | 1 (0.8) | 1 (0.8) | – | 2 (1.5) | 1 (0.8) | – | 5 (3.8) |
| Stroke, n (%) | 1 (0.8) | 1 (0.8) | 5 (3.8) | – | 1 (0.8) | – | 8 (6.1) |
| Respiratory failure with tracheostomy, n (%) | 4 (3.0) | 3 (2.3) | 3 (2.3) | 2 (1.5) | – | – | 12 (9.1) |
| Renal failure requiring dialysis, n (%) | – | 1 (0.8) | 4 (3.0) | – | 1 (0.8) | – | 6 (4.5) |
In each column, each individual patient is listed repeatedly for each of the multiple complications experienced; that is, the total number of patients with complications is less than the additive number in each column. DTAA, descending thoracic aortic aneurysm; TAAA, thoracoabdominal aortic aneurysm.
Within our study cohort, nine patients encountered SCI. Among them, five (3.8%) experienced transient lower extremity weakness, while four (3.0%) suffered persistent paraplegia, two of whom ultimately succumbed to complications during the hospital stay. Additionally, one patient with a contained rupture of a large type I TAAA developed lower extremity weakness on the fifth day after TAAA repair, which progressively advanced to complete paraplegia. Previously, the CSFD was reinserted upon the detection of weakness, revealing normal spinal fluid pressure. CT scans of the head did not indicate acute intracranial hemorrhage, territorial infarct, or hydrocephalus. Furthermore, eight patients (6.1%) experienced a stroke, twelve (9.1%) developed respiratory failure requiring tracheostomy, and six (4.5%) encountered renal failure necessitating dialysis. These complications were predominantly observed in patients with Crawford type I or II aneurysms, the most extensive type. The average length of hospital stay following surgery was 12.2±11.2 days.
Amongst the patients, in 25 (19%) some type of CSFD-related complication was reported, as detailed in Table 5. Persistent CSF leakage was experienced by nine patients (7%), with six of them also reporting headaches. Acetaminophen was used to manage headaches in most cases, with occasional use of caffeine, tramadol, or ketorolac, if acetaminophen was ineffective. In instances where spinal headaches persisted for 48 to 72 hours despite conservative measures, a CSF blood patch was employed. Suturing the insertion site or applying additional dressing helped control leakage in five and four patients, respectively. One patient (0.8%) with persistent CSF leakage and headache was found to have a spinal cutaneous fistula on the seventh postoperative day, which was sealed with a single stitch without any further complications.
Table 5
| CSFD complication | N [%] |
|---|---|
| Persistent CSF leakage | 9 [7] |
| Blood-tinged CSF | 14 [11] |
| Spinal cutaneous fistula | 1 [1] |
| Subdural hematoma | 3 [2] |
CSFD, cerebrospinal fluid drainage; CSF, cerebrospinal fluid.
Blood-tinged or pinkish CSF was observed in 14 patients (11%), with 12 of them not experiencing any neurological symptoms. Clarity of their CSF improved over time. In cases of distinctly bloody taps, elective aortic procedures were delayed, avoiding introducing blood into the spinal canal upon heparinization. Among the patients with blood-tinged CSF, three (2%) underwent a head CT scan, revealing no abnormality, and one of these patients also had a concomitant leakage with headache. Another patient with bloody CSF on 2nd postoperative day failed to regain consciousness. The CSFD was removed on the third postoperative day, and a brain CT performed on the thirteenth day revealed a subdural hemorrhage. Both the blood in the CSF and the intracranial hemorrhage were attributed to postoperative active coagulopathy. Unfortunately, the patient passed away on the fifteenth postoperative day. Additionally, another patient with persistent leakage was found to have a concomitant subdural hematoma during CT imaging. One patient had a subdural hematoma without any associated symptoms. None of the patients in our study required surgical intervention due to complications associated with CSFD.
Discussion
Earlier studies indicated no significant impact of CSF drain usage on the incidence of SCI (18), but since then, multiple studies, both retrospective and prospective, have yielded positive results regarding the efficacy of spinal drainage (19-21). The mechanism of this protective effect is thought to be related to reduction in intrathecal pressure, allowing greater spinal cord blood flow by decreasing the pressure on the cord itself (spinal cord perfusion pressure equals mean arterial pressure minus spinal cord fluid pressure) (19-25). However, the use of a spinal drain is not without complications of its own (26). These complications can be associated with the placement, dwelling, and removal of the drain, as well as the drainage itself of CSF. Complications can vary from minor inconveniences such as bloody spinal fluid and puncture-site bleeding, to major events like epidural hematoma, intracranial hemorrhage, subarachnoid hemorrhage, meningitis, and catheter/drainage-related neurological deficit. The reported complications also include hypotension, spinal headache, CSF leak requiring intervention (i.e., blood patch or suturing), drain fracture that may necessitate surgical removal, and occluded/dislodged catheters (14,15,26).
In a recent meta-analysis done by the Cornell group, CSF drain was found to be associated with decreased operative mortality in patients undergoing open repair of DTAA and TAAA (27). Another meta-analysis also showed that studies reporting greater CSF drain use had lower pooled permanent SCI rates compared with studies reporting lower use of CSF drains (28). A meta-analysis done by Khan et al. demonstrated decrease in the occurrence of SCI by half with the use of CSFD in open thoracoabdominal aortic repair (25). Our single-center study endorses the protective nature of CSFD as a key element of the SCI prevention armamentarium: only 3% of patients among a sizable number of open DTAA and TAAA aortic repairs developed permanent paraplegia, congruent with contemporary clinical outcomes (28,29). Use of a CSF drain was associated with complications in 19% of the patients, which were minor and without major neurological sequalae. In light of recent evidence, The American College of Cardiology and American Heart Association have updated the recommendation for spinal cord drainage from class IB (30) to class IA (12) in order to decrease the incidence of temporary as well as permanent SCI in patients undergoing open thoracoabdominal aortic repair.
Limitations
Our study is a single-center, retrospective investigation that does not include utilization of spinal drains in patients who did not have an open repair of their thoracic and thoracoabdominal aortas. This study also does not inform as to the difference between use and lack of use of CSF drains.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arora L, Hosn MA. Spinal cord perfusion protection for thoraco-abdominal aortic aneurysm surgery. Curr Opin Anaesthesiol 2019;32:72-9. [Crossref] [PubMed]
- Coselli JS, Green SY, Price MD, et al. Spinal cord deficit after 1114 extent II open thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2020;159:1-13. [Crossref] [PubMed]
- Etz CD, Weigang E, Hartert M, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgery†. Eur J Cardiothorac Surg 2015;47:943-57. [Crossref] [PubMed]
- Griepp EB, Di Luozzo G, Schray D, et al. The anatomy of the spinal cord collateral circulation. Ann Cardiothorac Surg 2012;1:350-7. [PubMed]
- First International Meeting: Eliminating Paralysis After Aortic Aneurysm Surgery. Ohio State University; 2023.
- Elsayad-Awad H. "Eliminating Paraplegia". Ohio State University Seminar. March 4, 2023.
- Ziganshin BA, Elefteriades JA. Surgical management of thoracoabdominal aneurysms. Heart 2014;100:1577-82. [Crossref] [PubMed]
- Abdelbaky M, Papanikolaou D, Zafar MA, et al. Safety of perioperative cerebrospinal fluid drain as a protective strategy during descending and thoracoabdominal open aortic repair. JTCVS Tech 2021;6:1-8. [Crossref] [PubMed]
- Abdelbaky M, Zafar MA, Saeyeldin A, et al. Routine anterior spinal artery visualization prior to descending and thoracoabdominal aneurysm repair: High detection success. J Card Surg 2019;34:1563-8. [Crossref] [PubMed]
- Ellauzi H, Arora H, Elefteriades JA, et al. Cerebrospinal Fluid Drainage for Prevention of Spinal Cord Ischemia in Thoracic Endovascular Aortic Surgery-Pros and Cons. Aorta (Stamford) 2022;10:290-7. [Crossref] [PubMed]
- Schachner T, Gottardi R, Schmidli J, et al. Practice of neuromonitoring in open and endovascular thoracoabdominal aortic repair-an international expert-based modified Delphi consensus study. Eur J Cardiothorac Surg 2023;63:ezad198. [Crossref] [PubMed]
- Isselbacher EM, Preventza O, Hamilton Black J 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022;146:e334-482. [Crossref] [PubMed]
- Riley SP, Donnelly MJ, Khatib D, et al. Post-dural puncture headaches following spinal drain placement during thoracoabdominal aortic aneurysm repair: incidence, associated risk factors, and treatment. J Anesth 2015;29:544-50. [Crossref] [PubMed]
- Youngblood SC, Tolpin DA, LeMaire SA, et al. Complications of cerebrospinal fluid drainage after thoracic aortic surgery: a review of 504 patients over 5 years. J Thorac Cardiovasc Surg 2013;146:166-71. [Crossref] [PubMed]
- Rong LQ, Kamel MK, Rahouma M, et al. Cerebrospinal-fluid drain-related complications in patients undergoing open and endovascular repairs of thoracic and thoraco-abdominal aortic pathologies: a systematic review and meta-analysis. Br J Anaesth 2018;120:904-13. [Crossref] [PubMed]
- Parotto M, Ouzounian M, Djaiani G. Spinal Cord Protection in Elective Thoracoabdominal Aortic Procedures. J Cardiothorac Vasc Anesth 2019;33:200-8. [Crossref] [PubMed]
- Peterss S, Charilaou P, Ziganshin BA, et al. Assessment of survival in retrospective studies: The Social Security Death Index is not adequate for estimation. J Thorac Cardiovasc Surg 2017;153:899-901. [Crossref] [PubMed]
- Crawford ES, Svensson LG, Hess KR, et al. A prospective randomized study of cerebrospinal fluid drainage to prevent paraplegia after high-risk surgery on the thoracoabdominal aorta. J Vasc Surg 1991;13:36-45; discussion 45-6.
- Safi HJ, Hess KR, Randel M, et al. Cerebrospinal fluid drainage and distal aortic perfusion: reducing neurologic complications in repair of thoracoabdominal aortic aneurysm types I and II. J Vasc Surg 1996;23:223-8; discussion 229. [Crossref] [PubMed]
- Estrera AL, Sheinbaum R, Miller CC, et al. Cerebrospinal fluid drainage during thoracic aortic repair: safety and current management. Ann Thorac Surg 2009;88:9-15; discussion 15. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9. [Crossref] [PubMed]
- Estrera AL, Miller CC 3rd, Huynh TT, et al. Preoperative and operative predictors of delayed neurologic deficit following repair of thoracoabdominal aortic aneurysm. J Thorac Cardiovasc Surg 2003;126:1288-94. [Crossref] [PubMed]
- Safi HJ, Bartoli S, Hess KR, et al. Neurologic deficit in patients at high risk with thoracoabdominal aortic aneurysms: the role of cerebral spinal fluid drainage and distal aortic perfusion. J Vasc Surg 1994;20:434-44; discussion 442-3. [Crossref] [PubMed]
- Estrera AL, Sandhu HK, Charlton-Ouw KM, et al. A Quarter Century of Organ Protection in Open Thoracoabdominal Repair. Ann Surg 2015;262:660-8. [Crossref] [PubMed]
- Khan NR, Smalley Z, Nesvick CL, et al. The use of lumbar drains in preventing spinal cord injury following thoracoabdominal aortic aneurysm repair: an updated systematic review and meta-analysis. J Neurosurg Spine 2016;25:383-93. [Crossref] [PubMed]
- Wynn MM, Sebranek J, Marks E, et al. Complications of spinal fluid drainage in thoracic and thoracoabdominal aortic aneurysm surgery in 724 patients treated from 1987 to 2013. J Cardiothorac Vasc Anesth 2015;29:342-50. [Crossref] [PubMed]
- Khan FM, Naik A, Hameed I, et al. Open Repair of Descending Thoracic and Thoracoabdominal Aortic Aneurysms: A Meta-Analysis. Ann Thorac Surg 2020;110:1941-9. [Crossref] [PubMed]
- Gaudino M, Khan FM, Rahouma M, et al. Spinal cord injury after open and endovascular repair of descending thoracic and thoracoabdominal aortic aneurysms: A meta-analysis. J Thorac Cardiovasc Surg 2022;163:552-64. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2016;151:1323-37. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]






